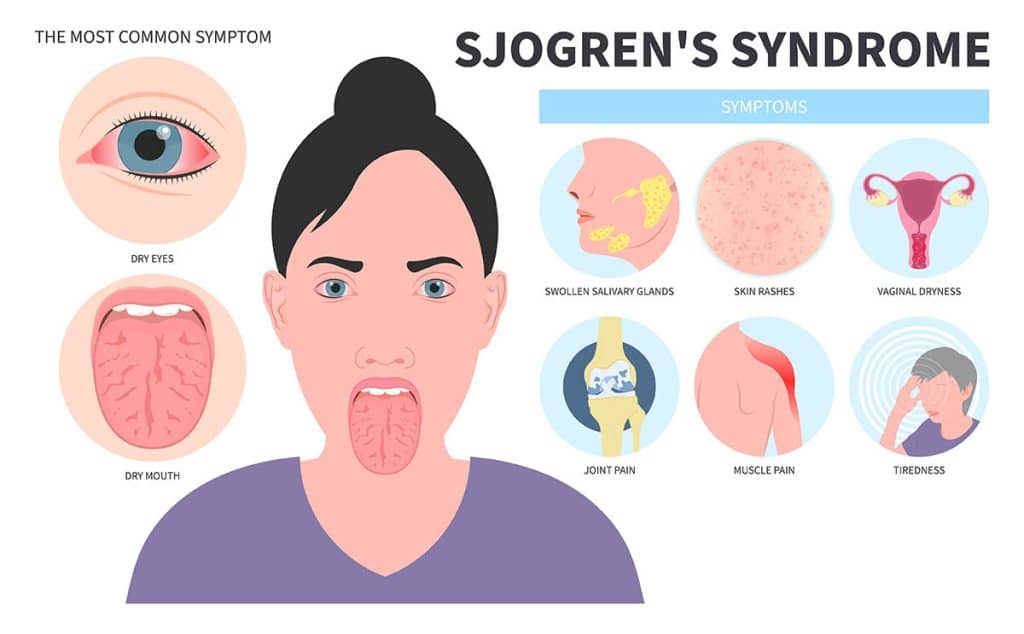
Xerostomia secondary to Sjögren’s syndrome is a hallmark manifestation of this chronic, systemic autoimmune disease, which primarily targets exocrine glands, especially the salivary and lacrimal glands. This results in significant salivary gland hypofunction, leading to persistent dry mouth and a cascade of oral health complications.
The pathogenesis of xerostomia in Sjögren’s syndrome involves lymphocytic infiltration, acinar cell destruction, and eventual fibrosis of the salivary glands, impairing saliva production and secretion.
Pathophysiology of Sjögren’s-Associated Xerostomia
In primary and secondary Sjögren’s syndrome, CD4+ T lymphocytes infiltrate the salivary glands, leading to:
- Apoptosis of acinar and ductal epithelial cells
- Autoantibody formation against Ro/SSA and La/SSB antigens
- Progressive glandular atrophy and fibrosis
- Cytokine-mediated inflammation (IL-6, TNF-α, IFN-γ)
This autoimmune cascade disrupts both volume and composition of saliva, reducing essential protective enzymes and buffering capacity, thus predisposing patients to oral diseases.
Key Clinical Features of Xerostomia in Sjögren’s Syndrome
Oral and Systemic Symptoms
- Persistent dry mouth despite fluid intake
- Difficulty in chewing, swallowing, and speaking
- Oral burning sensation, particularly on the tongue
- Dysgeusia (altered taste perception)
- Increased dental caries, especially root caries
- Recurrent oral candidiasis
- Parotid gland enlargement in some patients
These symptoms severely affect quality of life, nutritional status, and psychosocial well-being, often progressing insidiously.
Diagnostic Evaluation of Autoimmune Xerostomia
1. Clinical Assessment
- Duration and severity of dry mouth symptoms
- Associated dryness of eyes (keratoconjunctivitis sicca)
- Systemic manifestations (fatigue, arthralgia, rash)
2. Objective Salivary Gland Testing
- Sialometry: Unstimulated flow < 0.1 mL/min indicates severe hypofunction
- Sialography: “Fruit-laden, branchless tree” pattern in advanced cases
- Scintigraphy: Poor uptake and delayed excretion of radionuclide
3. Serological Markers
- Anti-Ro/SSA and Anti-La/SSB antibodies
- Elevated ANA, RF, and hypergammaglobulinemia
4. Minor Salivary Gland Biopsy
- Performed from the lower lip mucosa
- Focus score ≥ 1 (≥50 lymphocytes per 4 mm² of glandular tissue) confirms diagnosis
Differential Diagnosis
It is essential to rule out other causes of xerostomia:
- Radiation therapy (head and neck)
- Medications (anticholinergics, antidepressants)
- Hepatitis C, HIV-associated salivary gland disease
- Diabetes mellitus
- Sarcoidosis
- Amyloidosis
Management of Xerostomia Secondary to Sjögren’s Syndrome
Effective treatment requires symptom control, oral health preservation, and immune modulation.
1. Salivary Stimulants (Sialogogues)
Pilocarpine
- Muscarinic agonist; stimulates residual acinar cells
- 5 mg TID; side effects include sweating, nausea, and dizziness
Cevimeline
- More selective M3 receptor agonist
- 30 mg TID; fewer CNS-related side effects
Both drugs require functional gland tissue to be effective.
2. Saliva Substitutes
- Artificial saliva sprays or gels (e.g., carboxymethylcellulose-based)
- Mouth-coating agents for overnight use
- Glycerin-based lozenges for daytime relief
3. Immunomodulatory Therapy
For systemic manifestations and severe glandular dysfunction:
- Hydroxychloroquine: for arthralgia, fatigue, and mild disease
- Rituximab: anti-CD20 monoclonal antibody, used in refractory cases
- Corticosteroids or Methotrexate: in extraglandular involvement
Oral Health Management in Sjögren’s Xerostomia
Preventive Dental Care
- Daily application of 1.1% neutral sodium fluoride gel
- Avoidance of alcohol-based mouthwashes
- Regular professional dental cleaning every 3–6 months
Treatment of Opportunistic Infections
- Topical antifungals (nystatin or clotrimazole) for candidiasis
- Consider systemic fluconazole for recurrent infections
Diet and Lifestyle Adjustments
- Encourage frequent sipping of water
- Use of sugar-free xylitol-containing gum or lozenges
- Avoid caffeine, alcohol, spicy or dry foods
- Use of bedside humidifiers
Nutritional and Psychological Considerations
Patients often report malnutrition, social isolation, and depression due to persistent xerostomia. Multidisciplinary care involving rheumatologists, dentists, nutritionists, and psychologists is essential for optimal outcomes.
Emerging Therapies and Research Directions
1. Gene Therapy
- Introduction of aquaporin-1 gene into salivary duct cells
- Promotes water transport and improves saliva output
2. Mesenchymal Stem Cell Therapy
- Potential to regenerate damaged salivary gland tissues
- Early-phase trials showing promising results
3. Targeted Biologics
- Belimumab, abatacept, and tocilizumab under investigation
- Aim to modulate B-cell activation and cytokine activity
Prognosis and Long-Term Outlook
Sjögren’s syndrome is a chronic condition with no current cure. However, early diagnosis, regular monitoring, and individualized treatment plans can help manage xerostomia effectively and prevent serious complications like:
- Tooth loss
- Recurrent parotitis
- Non-Hodgkin’s lymphoma (increased lifetime risk)
Frequently Asked Questions:
Q1: Is xerostomia always present in Sjögren’s syndrome?
Yes, it is one of the primary clinical symptoms and often appears early.
Q2: Can xerostomia be reversed in Sjögren’s syndrome?
Complete reversal is rare, but symptoms can be managed effectively with medication and supportive care.
Q3: Are artificial saliva products effective?
They provide temporary relief and are useful when natural salivary function is absent.
Q4: Is dental decay inevitable in Sjögren’s-related xerostomia?
No, with proper preventive care, decay can be significantly reduced.
Q5: What systemic risks are associated with Sjögren’s?
Besides xerostomia, patients may develop renal, pulmonary, and neurological involvement, and have an elevated risk of lymphoma.
Xerostomia secondary to Sjögren’s syndrome is a complex, systemic manifestation with profound implications for oral and general health. By combining immunologic control, symptomatic management, and comprehensive oral care, we can mitigate the impact of this chronic autoimmune condition. Continued research into novel biologics, gene therapy, and regenerative medicine holds promise for improving outcomes and quality of life for affected individuals.