Colorectal cancer (CRC) remains a leading cause of cancer-related morbidity and mortality worldwide. In recent years, molecular diagnostics have transformed CRC treatment, particularly through identification of biomarkers such as epidermal growth factor receptor (EGFR) expression and wild-type KRAS status. This combination—wild-type KRAS, EGFR-positive colorectal cancer—defines a patient subset highly responsive to EGFR-targeted therapies, offering a pathway to improved survival through precision oncology.
Molecular Basis of EGFR and KRAS in Colorectal Cancer
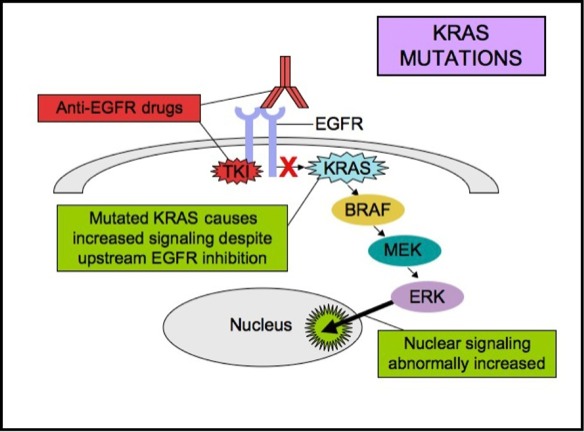
EGFR is a transmembrane tyrosine kinase receptor that drives tumorigenesis via the RAS–RAF–MEK–ERK and PI3K–AKT pathways. Activation leads to cellular proliferation, angiogenesis, and inhibition of apoptosis. However, mutations in downstream effectors—particularly in KRAS—can bypass EGFR inhibition, rendering anti-EGFR therapies ineffective.
Wild-Type KRAS Explained
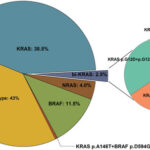
- Wild-type KRAS refers to the absence of activating mutations in exons 2, 3, and 4 of the KRAS gene.
- Only wild-type KRAS tumors retain the capability to respond to EGFR-targeted monoclonal antibodies.
EGFR Positivity in CRC
- EGFR overexpression, identified via immunohistochemistry (IHC), correlates with tumor aggressiveness.
- While EGFR positivity alone is insufficient for predicting response to EGFR therapy, combined wild-type KRAS and EGFR expression stratifies patients for optimal therapeutic benefit.
Rationale for Combined EGFR and KRAS Testing in CRC
EGFR expression by itself is not a reliable biomarker for anti-EGFR therapy because many tumors overexpress EGFR but harbor KRAS mutations that drive ligand-independent growth. Therefore, identifying patients with EGFR-positive, wild-type KRAS colorectal cancer is essential for guiding therapy.
Testing Modalities
- EGFR testing: IHC (standardized scoring methods under development)
- KRAS genotyping: PCR, Sanger sequencing, NGS platforms
Dual testing ensures therapy is directed only at those who can benefit, reducing toxicity and cost for patients unlikely to respond.
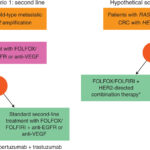
Anti-EGFR Therapy in EGFR-Positive Wild-Type KRAS CRC
The cornerstone of therapy for this subgroup is monoclonal antibody inhibition of EGFR, most commonly using cetuximab or panitumumab. These agents block ligand binding, suppressing downstream mitogenic signaling.
Recommended Regimens
| Therapy Line | Drug | Regimen | Survival Benefit |
|---|---|---|---|
| First-Line | Cetuximab + FOLFIRI/FOLFOX | Combination chemotherapy | Improved OS and PFS |
| Second-Line | Panitumumab | Monotherapy or combination | Disease control in prior chemo |
| Refractory | EGFR re-challenge trials | With or without MEK inhibitors | Investigational benefits |
Tumor Sidedness Impact
- Left-sided tumors demonstrate higher response rates.
- Right-sided CRC, even with wild-type KRAS and EGFR positivity, shows diminished benefit.
Prognostic and Predictive Value of Wild-Type KRAS and EGFR Status
Patients with wild-type KRAS and EGFR-positive tumors represent a favorable prognostic group, showing:
- Higher response rates to EGFR inhibition (RR > 60% in some studies)
- Longer median progression-free survival
- Improved overall survival, particularly in left-sided tumors
This biomarker profile also enables participation in biomarker-driven clinical trials, expanding access to emerging targeted agents.
Resistance Mechanisms in Wild-Type KRAS, EGFR-Positive CRC
Despite initial response, resistance often emerges during EGFR blockade. This is typically due to:
- Acquired KRAS mutations
- EGFR S492R extracellular mutations
- MET amplification or HER2 activation
- BRAF V600E mutations emerging under selective pressure
Liquid biopsies (ctDNA) are now being used to monitor emergence of resistance, allowing adaptive therapy modification.
The Role of Additional Biomarkers
For a complete molecular profile, clinicians should test for:
| Biomarker | Role | Testing Method |
|---|---|---|
| NRAS | Same pathway as KRAS; also must be wild-type | NGS or PCR |
| BRAF | V600E associated with poor outcomes | NGS, PCR |
| MSI/MMR | Predictive for immunotherapy response | IHC, PCR |
| HER2 | May indicate EGFR resistance; HER2-targetable | IHC, FISH |
A comprehensive panel improves the accuracy of precision therapy selection and enhances outcomes in complex metastatic cases.
Future Directions in Wild-Type KRAS, EGFR-Positive CRC
Personalized Combinations
- Trials combining EGFR inhibitors with MEK, PI3K, or immune checkpoint inhibitors are underway.
- Novel agents such as amivantamab (bispecific EGFR/MET antibody) are in clinical development.
Molecular Imaging and AI
Emerging use of radiogenomic profiling and AI-powered decision models may soon enhance biomarker selection and therapy sequencing in clinical practice.
Rechallenge Strategies
In patients who develop resistance after initial response, reintroduction of EGFR inhibitors following a therapy break has shown promise when resistance mutations decline in circulating tumor DNA.
EGFR-positive, wild-type KRAS colorectal cancer defines a molecularly distinct and therapeutically actionable subgroup. Through robust biomarker testing and tailored treatment regimens, we can optimize clinical outcomes, extend survival, and pioneer a future driven by personalized medicine. As resistance mechanisms become better understood, next-generation strategies will further refine management for this evolving molecular phenotype.