WHIM syndrome (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis) is a rare, autosomal dominant primary immunodeficiency disorder caused primarily by gain-of-function mutations in the CXCR4 gene. It is characterized by recurrent bacterial infections, reduced levels of immunoglobulins, chronic warts due to HPV infection, and neutropenia linked to bone marrow abnormalities.
The syndrome presents early in life and leads to significant morbidity due to its impact on the immune system’s capacity to fight infections effectively.
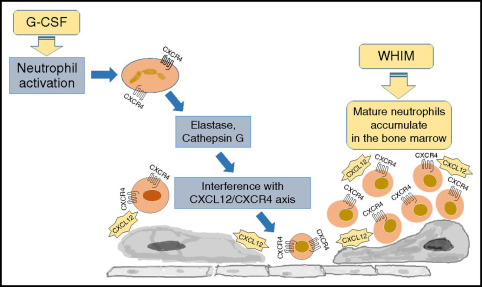
Genetic Basis and Pathophysiology of WHIM Syndrome
The defining genetic feature of WHIM syndrome is a mutation in the CXCR4 gene, which encodes a chemokine receptor crucial for immune cell trafficking. This mutation leads to hyperactive CXCR4 signaling, causing immune cells, particularly neutrophils, to become abnormally retained within the bone marrow—a phenomenon known as myelokathexis.
Key Pathophysiological Mechanisms:
- CXCR4 Gain-of-Function: Enhances retention signals in bone marrow
- Myelokathexis: Neutrophil maturation occurs, but cells fail to enter circulation
- Hypogammaglobulinemia: Deficient antibody production due to impaired B-cell function
- Susceptibility to HPV: Inadequate immune surveillance fosters wart development
Clinical Manifestations and Diagnostic Criteria
WHIM syndrome is often diagnosed based on a characteristic clinical tetrad and supported by genetic testing.
Core Clinical Features:
- Warts: Extensive and treatment-resistant HPV-induced lesions
- Hypogammaglobulinemia: Low levels of IgG, IgA, and/or IgM
- Recurrent Bacterial Infections: Particularly sinopulmonary infections like otitis media and pneumonia
- Myelokathexis: Neutropenia with mature neutrophils trapped in the bone marrow
Additional Symptoms:
- Delayed wound healing
- Chronic sinusitis
- Lymphopenia
- Failure to thrive in children
Diagnostic Tools:
- Complete Blood Count (CBC): Neutropenia, lymphopenia
- Bone Marrow Biopsy: Hypercellularity with neutrophil retention
- Immunoglobulin Quantification: IgG, IgA, IgM deficiency
- Flow Cytometry: Lymphocyte subset analysis
- Genetic Testing: Detection of CXCR4 mutations confirms diagnosis
Immunological Abnormalities in WHIM Syndrome
WHIM syndrome encompasses several immune deficits:
- Neutropenia: Due to sequestration, not production failure
- Hypogammaglobulinemia: Often moderate but clinically significant
- Lymphopenia: Reduced T and B cell counts
- Poor Antibody Response: Inefficient vaccine responses
- Deficient Dendritic Cell Trafficking: Impairs antigen presentation
Recurrent Infections: A Persistent Challenge
Patients frequently suffer from:
- Respiratory Infections: Otitis media, bronchitis, sinusitis, pneumonia
- Skin Infections: Due to bacterial or viral pathogens
- HPV-Related Lesions: Genital warts, plantar warts, and extensive cutaneous warts
Persistent infections contribute to progressive tissue damage and increase the risk of secondary complications such as bronchiectasis or malignancies (notably HPV-induced cancers).
Current Treatment Approaches for WHIM Syndrome
Effective management of WHIM syndrome requires a multidisciplinary strategy focusing on infection prevention, immune support, and symptom control.
1. Immunoglobulin Replacement Therapy (IVIG/SCIG)
- Mitigates hypogammaglobulinemia
- Reduces infection frequency
- Requires regular dosing intervals
2. G-CSF (Granulocyte Colony-Stimulating Factor)
- Mobilizes neutrophils from the bone marrow
- Alleviates neutropenia and improves infection resistance
3. Antiviral and Antibacterial Prophylaxis
- Long-term antibiotics for chronic bacterial infections
- HPV vaccinations and antiviral agents to limit wart progression
4. Targeted Therapies (CXCR4 Antagonists)
- Plerixafor (AMD3100): A promising drug that disrupts CXCR4-CXCL12 signaling, releasing neutrophils and lymphocytes into circulation
- Demonstrates potential as a disease-modifying agent
5. Hematopoietic Stem Cell Transplantation (HSCT)
- Considered in severe or refractory cases
- May provide a curative outcome, though with risks
Prognosis and Long-Term Outlook
WHIM syndrome is a lifelong condition, but early diagnosis and tailored treatment significantly improve outcomes. With consistent monitoring and immunological support, many patients live well into adulthood.
Prognostic Factors:
- Severity of neutropenia and infection frequency
- Response to immunoglobulin therapy
- Extent of HPV burden
- Accessibility of emerging treatments like plerixafor
Emerging Research and Future Directions
Ongoing research focuses on:
- Novel CXCR4 Inhibitors: Long-acting, orally bioavailable formulations
- Gene Editing: Targeting CXCR4 mutations using CRISPR-Cas9
- HPV Vaccine Strategies: For prevention in immunocompromised individuals
- Longitudinal Registries: To better understand disease progression and treatment outcomes
Frequently Asked Questions:
What is WHIM syndrome?
A rare immunodeficiency disorder marked by warts, hypogammaglobulinemia, infections, and myelokathexis caused by CXCR4 gene mutations.
Is WHIM syndrome inherited?
Yes, it follows an autosomal dominant inheritance pattern but can also arise from de novo mutations.
How is WHIM syndrome treated?
Primarily with immunoglobulin therapy, G-CSF, antibiotics, and CXCR4 antagonists like plerixafor. In some cases, stem cell transplantation is considered.
What causes warts in WHIM syndrome?
Persistent HPV infections, due to impaired immune control, lead to extensive and chronic warts.
Can WHIM syndrome be cured?
There is no definitive cure, but long-term management can control symptoms. HSCT may offer a potential cure in select patients.
How common is WHIM syndrome?
It is extremely rare, with fewer than 100 cases reported worldwide. However, underdiagnosis is possible due to symptom overlap with other immunodeficiencies.
WHIM syndrome exemplifies the complexity of primary immunodeficiencies. By understanding its genetic basis, immune dysfunctions, and clinical manifestations, we can deliver more accurate diagnoses and improve therapeutic outcomes. Advances in targeted therapies and early intervention strategies continue to offer hope for individuals affected by this rare but significant disorder.

