Vitamin D Dependent Rickets (VDDR) is a rare group of genetic disorders that impair the metabolism or function of vitamin D, leading to defective bone mineralization and skeletal deformities. Unlike nutritional rickets, which arises from inadequate dietary intake or insufficient sunlight exposure, VDDR stems from intrinsic metabolic abnormalities affecting the synthesis, activation, or response to vitamin D.
VDDR is classified primarily into two major types, with subcategories based on genetic and enzymatic defects. Early recognition and proper treatment are essential to prevent permanent skeletal deformities and optimize long-term outcomes.
Types of Vitamin D Dependent Rickets
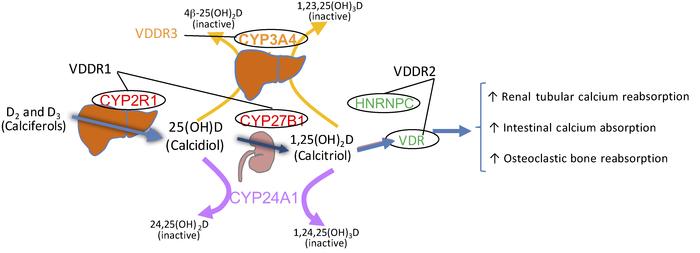
VDDR Type 1A (1α-hydroxylase deficiency)
- Cause: Mutations in the CYP27B1 gene impair 1α-hydroxylase activity.
- Pathophysiology: Inability to convert 25-hydroxyvitamin D [25(OH)D] into its active form, 1,25-dihydroxyvitamin D [1,25(OH)₂D].
- Inheritance: Autosomal recessive.
- Clinical Features:
- Hypocalcemia
- Hypophosphatemia
- Elevated parathyroid hormone (PTH)
- Normal or elevated 25(OH)D levels
- Low or undetectable 1,25(OH)₂D levels
VDDR Type 1B (25-hydroxylase deficiency)
- Cause: Mutations in the CYP2R1 gene result in deficient 25-hydroxylase.
- Pathophysiology: Impaired conversion of vitamin D into 25(OH)D in the liver.
- Inheritance: Autosomal recessive.
- Clinical Features:
- Severe vitamin D deficiency despite supplementation
- Low 25(OH)D and 1,25(OH)₂D
- Similar skeletal manifestations as in VDDR1A
VDDR Type 2A (Hereditary Vitamin D-Resistant Rickets)
- Cause: Mutations in the VDR gene affecting the vitamin D receptor.
- Pathophysiology: Target tissues are resistant to 1,25(OH)₂D.
- Inheritance: Autosomal recessive.
- Clinical Features:
- Alopecia (in ~50% of cases)
- Hypocalcemia, hypophosphatemia
- Elevated 1,25(OH)₂D due to end-organ resistance
VDDR Type 2B
- Cause: Defective expression of the vitamin D receptor coactivator proteins.
- Clinical Features: Similar to VDDR2A but without identifiable VDR gene mutations.
Clinical Presentation
- Bone pain and tenderness
- Delayed growth and short stature
- Muscle weakness
- Genu valgum (knock-knees) or genu varum (bowlegs)
- Delayed walking or crawling
- Rachitic rosary (prominent costochondral junctions)
- Dental abnormalities
- Seizures (due to hypocalcemia)
In VDDR2A, alopecia may appear early in life and is a key diagnostic clue.
Diagnostic Evaluation
Laboratory Investigations
| Test | VDDR1A | VDDR1B | VDDR2A/B |
|---|---|---|---|
| Serum calcium | ↓ | ↓ | ↓ |
| Serum phosphate | ↓ | ↓ | ↓ |
| Alkaline phosphatase | ↑ | ↑ | ↑ |
| PTH | ↑ | ↑ | ↑ |
| 25(OH)D | Normal or ↑ | ↓ | Normal or ↑ |
| 1,25(OH)₂D | ↓ | ↓ | ↑ |
Genetic Testing
Definitive diagnosis is established via molecular genetic testing identifying mutations in CYP27B1, CYP2R1, or VDR.
Imaging Findings
- Widened, cupped, and frayed metaphyses
- Looser zones (pseudofractures)
- Generalized osteopenia
- Delayed bone age
Radiographs of the wrist and knees are typically used to evaluate metaphyseal changes.
Treatment and Management
VDDR Type 1A and 1B
- Active vitamin D (Calcitriol or Alfacalcidol): Bypasses the enzymatic defect.
- Calcium supplements: To correct hypocalcemia.
- Monitoring: Regular assessment of calcium, phosphate, PTH, and renal function to prevent hypercalcemia and nephrocalcinosis.
VDDR Type 2A and 2B
- High-dose Calcitriol: Often needed in pharmacologic doses.
- Oral or intravenous calcium: May be required to bypass intestinal resistance.
- Alopecia management: Primarily supportive; no effective therapy exists for the hair loss.
Prognosis
Early diagnosis and consistent treatment yield excellent outcomes in VDDR1. Children often achieve normal growth and skeletal development with appropriate therapy. However, VDDR2 is more difficult to manage due to end-organ resistance and carries a higher risk of complications.
Delayed treatment in any type may result in:
- Permanent skeletal deformities
- Growth retardation
- Dental anomalies
- Chronic pain and disability
Prevention and Genetic Counseling
- Prenatal counseling: Recommended for families with a history of VDDR.
- Carrier screening: Possible through genetic testing.
- Newborn screening: May be considered in high-risk populations.
- Vitamin D supplementation: Not effective as a preventive measure for VDDR but important in nutritional rickets.
Vitamin D Dependent Rickets, though rare, requires a high index of suspicion, especially in children with rickets-like symptoms but normal or elevated levels of 25(OH)D. Early differentiation from nutritional rickets is vital to initiate the correct form of treatment and avoid long-term complications. Genetic insights continue to shape precise diagnostics and pave the way for individualized care protocols.