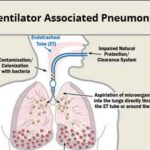
Ventilator-associated pneumonia (VAP) due to Acinetobacter species, particularly Acinetobacter baumannii, presents a formidable challenge in critical care settings. Known for its ability to colonize the respiratory tract and persist on hospital surfaces, Acinetobacter is a significant cause of nosocomial infections, especially in patients undergoing prolonged mechanical ventilation. Its multidrug-resistant (MDR) profile further complicates treatment strategies and contributes to elevated morbidity and mortality.
Pathogenesis of Acinetobacter-Related VAP
Acinetobacter baumannii exhibits a unique ability to survive in harsh conditions, making it a prevalent pathogen in the ICU environment. Colonization of the endotracheal tube and biofilm formation facilitate its entry into the lower respiratory tract.
Key Contributing Factors:
- Mechanical disruption of airway defenses
- Prolonged ventilation (>48 hours)
- Prior antibiotic exposure
- Cross-contamination via healthcare personnel and equipment
- Environmental persistence on dry surfaces
Microbiological Profile and Resistance Mechanisms
Characteristics of Acinetobacter baumannii:
- Gram-negative, aerobic, non-motile coccobacillus
- Resistant to desiccation and disinfectants
- Capable of rapid gene transfer leading to multidrug resistance
Common Resistance Mechanisms:
- Beta-lactamase production, including OXA-type carbapenemases
- Efflux pumps reducing antibiotic concentrations
- Porin modifications decreasing drug penetration
- Biofilm formation protecting against host immunity and antibiotics
Multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) strains are increasingly observed, particularly in regions with high antibiotic misuse.
Clinical Features and Diagnostic Criteria
VAP due to Acinetobacter often manifests with:
- Fever or hypothermia
- Leukocytosis or leukopenia
- Purulent respiratory secretions
- New or progressive infiltrates on chest radiographs
- Hypoxemia or increasing oxygen requirements
Diagnostic Modalities:
- Quantitative cultures from:
- Endotracheal aspirate (ETA)
- Bronchoalveolar lavage (BAL)
- Protected specimen brush (PSB)
- Blood cultures for detecting bacteremia
- Procalcitonin levels as adjunct for infection monitoring
- Molecular testing (PCR) to detect resistance genes (e.g., OXA, NDM)
Empirical and Targeted Treatment Strategies
Due to the high prevalence of MDR strains, treatment of Acinetobacter-associated VAP demands careful selection of antimicrobials based on local resistance profiles.
Empirical Therapy:
Initiate broad-spectrum coverage when VAP is suspected, especially in patients at high risk for MDR pathogens.
Recommended agents may include:
- Carbapenems (if susceptible)
- Ampicillin-sulbactam (high-dose regimens)
- Polymyxins (colistin or polymyxin B) for carbapenem-resistant strains
- Tigecycline (in combination for salvage therapy)
- Cefiderocol (novel siderophore cephalosporin, if available)
De-escalation Based on Culture:
Tailor antibiotic therapy as soon as culture and sensitivity results are available. Combination therapy may be necessary for XDR strains.
Duration of therapy: Typically 7 days, extended if clinical response is inadequate or complications arise.
Role of Inhaled Antibiotics
Inhaled (aerosolized) antibiotics, such as colistin or amikacin, can deliver high local concentrations to the lungs with reduced systemic toxicity. They are particularly useful in adjunct to systemic therapy for MDR Acinetobacter VAP.
Infection Control and Prevention Measures
Environmental and Contact Precautions:
- Use of single-use medical equipment when possible
- Rigorous disinfection protocols for ventilators and surfaces
- Contact isolation of colonized/infected patients
Ventilator Care Bundle Components:
- Elevation of the head of the bed (30°–45°)
- Daily sedation interruption and assessment for extubation
- Subglottic suctioning to reduce microaspiration
- Oral decontamination using chlorhexidine
- Minimizing unnecessary antibiotic use
Antimicrobial Stewardship:
- Empirical therapy based on local antibiograms
- Avoiding unnecessary broad-spectrum antibiotics
- Monitoring resistance trends over time
Emerging Therapies and Research Directions
Novel Agents:
- Cefiderocol: Demonstrates potent activity against MDR Acinetobacter
- Eravacycline: A tetracycline-class antibiotic with promising efficacy
- Monoclonal antibodies and bacteriophage therapy: Experimental options targeting resistant bacteria
Rapid Diagnostics:
Implementation of multiplex PCR and whole-genome sequencing in ICU settings allows real-time identification of resistance markers, facilitating targeted therapy.
Prognosis and Clinical Outcomes
Acinetobacter-related VAP is associated with higher mortality compared to other pathogens due to delays in effective therapy and limited treatment options. Mortality rates range from 30% to over 60% in ICU patients.
Factors influencing prognosis:
- Timeliness and appropriateness of antibiotic therapy
- Presence of septic shock or organ dysfunction
- Comorbidities such as COPD, diabetes, renal failure
- Resistance profile of the infecting strain
Ventilator-associated pneumonia due to Acinetobacter baumannii remains a formidable clinical challenge in intensive care settings. Its resilience, resistance patterns, and environmental persistence demand a multifaceted approach involving timely diagnosis, precision-guided antimicrobial therapy, strict infection control, and ongoing stewardship. Embracing innovation in diagnostics and therapeutics is key to improving patient outcomes and limiting the spread of resistant strains.