Uveal melanoma (UM) is the most common primary intraocular malignancy in adults, originating from melanocytes within the uveal tract, which includes the iris, ciliary body, and choroid. Despite successful local tumor control in over 90% of cases through radiation or surgical resection, up to 50% of patients eventually develop metastases, primarily to the liver. Prognosis post-metastasis remains poor, with median survival ranging from 6 to 12 months.
The immunobiology of UM differs significantly from cutaneous melanoma, particularly in its reduced mutational burden and immune-evasive characteristics. Notably, HLA-A*02:01 positivity plays a pivotal role in determining patient eligibility for targeted T-cell receptor (TCR) immunotherapies, marking a turning point in personalized cancer treatment.
HLA-A*02:01 in Uveal Melanoma: Immunogenetic Significance
HLA Typing and Antigen Presentation
HLA-A02:01 is a specific allele of the major histocompatibility complex (MHC) class I gene locus. This allele encodes a protein responsible for presenting intracellular peptides, including tumor-associated antigens, to cytotoxic CD8+ T cells. The presence of HLA-A02:01 in UM patients enables the use of engineered TCR therapies that specifically recognize antigenic peptides bound to this MHC molecule.
Frequency and Clinical Relevance
Approximately 40–50% of Caucasians express the HLA-A*02:01 allele, making it a critical selection criterion in clinical trials utilizing peptide-specific T-cell therapies. This subset of patients benefits from emerging therapies targeting tumor-specific antigens such as gp100, MART-1, and PRAME.
Molecular and Antigenic Landscape of Uveal Melanoma
Key Tumor-Associated Antigens
- gp100 (PMEL17): A melanosomal glycoprotein abundantly expressed in melanocytes and UM cells. The gp100 peptide (gp100:209–217) is presented by HLA-A*02:01 and is the target of the FDA-approved tebentafusp therapy.
- MART-1 (Melan-A): Another melanocyte differentiation antigen involved in T-cell recognition.
- PRAME (Preferentially Expressed Antigen in Melanoma): Overexpressed in metastatic UM and implicated in immune evasion.
Genetic Alterations
- GNAQ and GNA11 mutations: Present in over 85% of UM cases, contributing to MAPK pathway activation.
- BAP1 loss: Associated with metastatic potential and poor prognosis.
Diagnostic Evaluation and HLA Typing
Standard Diagnostic Workup
- Ophthalmoscopic examination
- Ultrasound biomicroscopy
- MRI or CT for extraocular extension
- Liver imaging (MRI preferred) due to high risk of hepatic metastasis
HLA Genotyping
Genotyping for HLA-A*02:01 is essential to stratify patients for antigen-specific therapies. This is typically performed using PCR-based techniques or next-generation sequencing of peripheral blood samples.
Immunotherapy in HLA-A*02:01-Positive Uveal Melanoma Patients
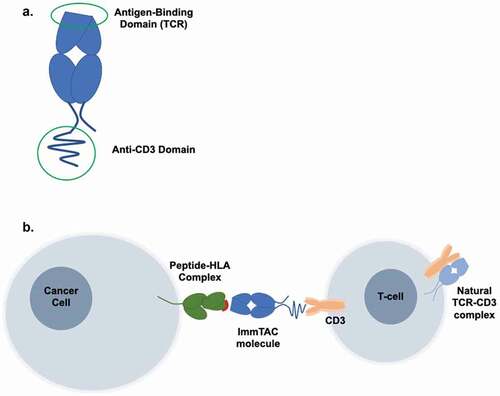
Tebentafusp (IMCgp100)
Tebentafusp is a bispecific T cell engager (ImmTAC) that targets the gp100 peptide-HLA-A*02:01 complex and recruits T cells via an anti-CD3 effector domain. It is the first and only approved systemic therapy shown to improve overall survival in metastatic UM.
| Therapeutic Target | Mechanism | Eligibility |
|---|---|---|
| gp100/HLA-A*02:01 | T cell redirection and activation against UM cells | HLA-A*02:01-positive only |
Engineered TCR-T Cell Therapy
Patients with confirmed HLA-A02:01 expression may also be candidates for autologous TCR-engineered T cell therapy. These T cells are genetically modified to express high-affinity TCRs specific to tumor antigens like MART-1 or PRAME presented in the context of HLA-A02:01.
Challenges and Immune Evasion Mechanisms
Tumor Microenvironment
UM exhibits a low mutational load and immunosuppressive tumor microenvironment, characterized by:
- Limited T-cell infiltration
- High expression of immune checkpoint molecules (e.g., PD-L1)
- TGF-β and IDO-mediated immunosuppression
Downregulation of HLA Expression
A subset of UM tumors may escape immune recognition by downregulating HLA class I molecules, thereby reducing antigen presentation, even in HLA-A*02:01-positive individuals.
Prognosis and Treatment Outcomes
| Patient Subgroup | Median Overall Survival | Notes |
|---|---|---|
| General metastatic UM | 6–12 months | Limited response to conventional chemotherapy |
| Tebentafusp-treated HLA-A*02:01+ UM | ~21.7 months | Significant survival advantage in clinical trials |
| HLA-A*02:01+ patients with TCR therapy | Under investigation | Early-phase trials show promising responses in heavily pretreated patients |
Future Directions in HLA-A*02:01-Targeted UM Therapy
Neoantigen Vaccines
Personalized vaccines leveraging the unique mutational profile of each UM tumor could be paired with HLA-A*02:01 presentation to stimulate an adaptive immune response.
Combination Immunotherapies
Clinical trials are ongoing to evaluate combinations of TCR therapy with checkpoint inhibitors, cytokine agonists, and agents that modulate the tumor microenvironment to enhance immune activation.
Biomarker Discovery
Further research is focused on identifying predictive biomarkers beyond HLA status to refine patient selection and predict durable response to immunotherapy.
Frequently Asked Questions:
What is the significance of HLA-A*02:01 in uveal melanoma?
HLA-A*02:01 is critical for presenting specific tumor antigens to cytotoxic T cells. Its presence allows the use of targeted therapies like tebentafusp and TCR-engineered T cell therapies.
Can patients without HLA-A*02:01 receive similar treatments?
Currently, tebentafusp and many TCR therapies are limited to HLA-A*02:01-positive patients due to MHC-restricted antigen presentation. Research is ongoing to expand these options.
What are the side effects of tebentafusp?
Common side effects include cytokine release syndrome (CRS), rash, and liver enzyme elevations. These are generally manageable with supportive care.
Is metastatic uveal melanoma curable?
While not curable in most cases, survival can be extended through targeted immunotherapy in selected patients, particularly those who are HLA-A*02:01-positive.
How is HLA-A*02:01 tested?
HLA typing is done through PCR or next-generation sequencing using blood samples. It is a routine part of workup for patients considered for antigen-specific immunotherapy.