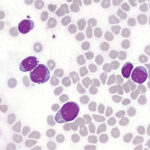
T-cell lymphoblastic lymphoma (T-LBL) is a rare, aggressive non-Hodgkin lymphoma characterized by the malignant proliferation of immature T-cell precursors. Closely related to T-cell acute lymphoblastic leukemia (T-ALL), T-LBL is distinguished by its predominant involvement of nodal and extranodal tissues, particularly the mediastinum, without significant bone marrow infiltration at the time of diagnosis.
Representing approximately 2% of adult non-Hodgkin lymphomas and up to 25% of pediatric lymphomas, T-LBL primarily affects adolescent males and young adults. Prompt and intensive treatment is crucial due to the rapid growth rate of this malignancy.
Pathogenesis and Cellular Characteristics
T-LBL arises from early-stage T-cell progenitors, typically originating in the thymus. These immature cells undergo malignant transformation due to various oncogenic events, leading to uncontrolled proliferation and lymphoid tissue infiltration.
Genetic and Molecular Abnormalities:
- NOTCH1 mutations (common in over 50% of cases)
- CDKN2A/2B deletions
- TAL1, LMO1/2 overexpression
- PTEN and FBXW7 mutations
- Translocations involving T-cell receptor genes
These abnormalities result in the dysregulation of cell cycle control, apoptosis, and T-cell differentiation, contributing to the highly proliferative nature of T-LBL.
Epidemiology and Risk Factors
T-LBL predominantly affects:
- Children and adolescents aged 10–20 years
- Males, with a 2–3:1 male-to-female ratio
- Immunocompromised individuals (rare association)
Although the etiology remains largely unknown, potential risk factors include exposure to ionizing radiation, inherited genetic predispositions, and previous chemotherapy or radiation for other malignancies.
Clinical Presentation and Common Symptoms
T-LBL typically presents with signs related to a rapidly growing mediastinal mass and systemic symptoms. The disease can also affect lymph nodes, pleura, pericardium, and occasionally the skin or central nervous system.
Key Clinical Features:
- Mediastinal mass causing cough, chest pain, dyspnea, or superior vena cava syndrome
- Lymphadenopathy, particularly cervical and supraclavicular
- Pleural or pericardial effusions
- Fever, night sweats, weight loss (B symptoms)
- Bone pain or organomegaly in advanced stages
- Central nervous system involvement (headache, cranial nerve palsy) in rare cases
Diagnostic Evaluation
Accurate diagnosis requires a comprehensive evaluation integrating imaging, histology, immunophenotyping, and molecular studies.
1. Imaging Studies
- Chest X-ray/CT Scan: Identifies mediastinal mass or lymphadenopathy
- PET-CT: Assesses metabolic activity and full-body involvement
- MRI/CT Brain and Spine: For CNS evaluation in high-risk patients
2. Tissue Biopsy
- Excisional or core needle biopsy of affected lymph node or mediastinal tissue
- Histological findings: Small-to-medium-sized blasts with high mitotic activity
3. Immunophenotyping by Flow Cytometry
- Typical T-LBL markers:
- CD3+, CD7+, CD2+, CD5+, TdT+, CD1a+, CD4+/CD8+ (double-positive or negative)
4. Bone Marrow Examination
- To distinguish from T-ALL: If bone marrow blast involvement ≥25%, diagnosis shifts to T-ALL
5. Cerebrospinal Fluid (CSF) Analysis
- Lumbar puncture to assess CNS infiltration
6. Cytogenetics and Molecular Testing
- Evaluates chromosomal abnormalities and MRD (minimal residual disease) status
Differential Diagnosis
T-LBL must be differentiated from:
- T-cell acute lymphoblastic leukemia (T-ALL)
- Other peripheral T-cell lymphomas
- Small round blue cell tumors (e.g., Ewing sarcoma)
- B-cell lymphoblastic lymphoma
Clear distinction aids in appropriate therapeutic planning.
Staging and Classification
The Ann Arbor staging system with Cotswolds modifications is used:
- Stage I: Single nodal region or single extralymphatic site
- Stage II: Two or more nodal regions on the same side of the diaphragm
- Stage III: Nodal involvement on both sides of the diaphragm
- Stage IV: Diffuse involvement of extranodal organs (e.g., bone marrow, CNS)
Treatment Strategies for T-Cell Lymphoblastic Lymphoma
T-LBL is treated with ALL-type chemotherapy regimens, often modeled after pediatric T-ALL protocols due to their superior efficacy.
1. Induction Chemotherapy
Objective: Achieve complete remission
Regimen: Vincristine, anthracycline (daunorubicin), corticosteroids, L-asparaginase
2. Consolidation Therapy
Regimen: High-dose methotrexate, cytarabine, cyclophosphamide
3. CNS Prophylaxis
- Intrathecal chemotherapy (methotrexate, cytarabine)
- Cranial irradiation only in select high-risk cases
4. Maintenance Therapy
- 12–24 months duration
- Oral 6-mercaptopurine, methotrexate, intermittent vincristine/steroids
5. Hematopoietic Stem Cell Transplantation (HSCT)
- Reserved for relapsed/refractory cases
- Considered in high-risk or poor MRD responders
Emerging Therapeutic Approaches
Recent developments aim to target specific molecular pathways in T-LBL.
Targeted Agents:
- NOTCH1 inhibitors (gamma-secretase inhibitors)
- PI3K/mTOR inhibitors
- BCL-2 inhibitors (venetoclax)
Immunotherapy:
- Anti-CD7, CD38 monoclonal antibodies
- CAR-T cell therapy (investigational in T-cell malignancies)
Clinical trials are ongoing to assess safety and efficacy of these therapies.
Prognosis and Survival Outcomes
Prognosis is largely determined by:
- Age and performance status
- Stage at diagnosis
- CNS or bone marrow involvement
- MRD status post-induction
Survival Rates:
| Patient Group | 5-Year Overall Survival |
|---|---|
| Pediatric Patients | 80–90% |
| Young Adults | 60–75% |
| Adults >40 years | 40–60% |
| Relapsed Patients | <20% (variable) |
Early treatment response, especially MRD negativity, is the strongest predictor of long-term survival.
Follow-Up and Long-Term Management
Post-treatment surveillance involves:
- Routine physical exams and blood tests
- Periodic imaging to detect recurrence
- Monitoring for late toxicities (cardiotoxicity, infertility, secondary malignancies)
- Psychosocial support and rehabilitation services
T-cell lymphoblastic lymphoma is a high-grade, rapidly progressing malignancy that shares biological and clinical characteristics with T-ALL. With intensive combination chemotherapy and evolving molecular strategies, outcomes—particularly in children and adolescents—have significantly improved. Precise diagnosis, risk-adapted therapy, and diligent follow-up are essential in optimizing long-term survival and quality of life for patients.