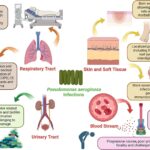
Cystic fibrosis (CF) is a genetic disorder characterized by defective chloride ion transport, leading to thickened mucus in the airways. This viscous environment predisposes patients to persistent respiratory infections, with Pseudomonas aeruginosa representing the most common and difficult-to-eradicate pathogen. Chronic colonization by P. aeruginosa significantly accelerates pulmonary decline, increases hospitalization frequency, and contributes to morbidity and mortality.
The pathophysiological niche created by the CF lung—marked by hypoxia, mucus accumulation, and inflammation—favors P. aeruginosa biofilm formation, antibiotic resistance, and immune evasion. Therefore, synergistic antibiotic combinations are indispensable in managing both early and chronic infections.
Pathogenesis and Resistance Mechanisms of P. aeruginosa in CF Airways
Once introduced into the CF lung, P. aeruginosa undergoes phenotypic changes that promote persistence, including the conversion to a mucoid phenotype driven by alginate production and enhanced quorum sensing activity. This transition supports the establishment of biofilms, which are dense, structured bacterial communities encased in extracellular polymeric substances.
Resistance mechanisms that complicate treatment include:
- Efflux pump overexpression (MexAB-OprM, MexXY-OprM)
- Loss of porins, such as OprD
- Induction of β-lactamases
- Hypermutability, fostering rapid evolution of resistance
- Anaerobic metabolism, reducing efficacy of oxygen-dependent antibiotics
These adaptations necessitate combination therapy capable of penetrating biofilms and overcoming metabolic dormancy.
The Rationale for Synergistic Antibiotic Therapy in CF
Synergistic therapy aims to amplify bacterial killing while minimizing the risk of resistance development. In the context of CF, such combinations are essential due to:
- Poor antibiotic penetration in sputum
- Polymicrobial infections
- Heterogeneous resistance within biofilms
- Chronic exposure to antimicrobials promoting adaptive resistance
Synergy is typically defined by in vitro assays showing a ≥2-log reduction in colony-forming units (CFU) when two agents are used in combination compared to the most active single agent.
Key Synergistic Antibiotic Combinations for P. aeruginosa in CF
β-Lactams and Aminoglycosides
β-lactams disrupt bacterial cell walls, allowing enhanced aminoglycoside entry and intracellular activity. Common regimens include:
- Ceftazidime + Tobramycin
- Meropenem + Amikacin
- Piperacillin-tazobactam + Tobramycin
These combinations are widely used during acute exacerbations and show robust synergy in time-kill studies and clinical practice.
Dual Inhaled Antibiotics
Inhaled therapy provides high local drug concentrations with minimal systemic toxicity. Synergistic inhaled regimens include:
- Tobramycin + Colistin
- Aztreonam lysine + Tobramycin
This approach improves lung function and reduces bacterial density in the sputum of chronically infected individuals.
Colistin-Based Synergy
Polymyxins like colistin target the bacterial membrane, increasing permeability and facilitating co-administered agents’ action. Effective pairings include:
- Colistin + Rifampin
- Colistin + Meropenem
- Colistin + Ciprofloxacin
Colistin’s nephrotoxicity necessitates therapeutic drug monitoring, particularly in systemic use.
Novel and Investigational Agents
- Ceftolozane-tazobactam + Amikacin: Exhibits synergy against MDR and XDR isolates.
- Ceftazidime-avibactam + Aztreonam: Potent against metallo-β-lactamase producers, a growing concern in CF pathogens.
- Phage therapy + Antibiotics: Engineered bacteriophages in combination with antibiotics show promise in compassionate use cases.
Biofilm-Targeted Synergy: Disrupting the Stronghold
Biofilms are inherently resistant to monotherapy due to their structural and metabolic defenses. Strategies to enhance penetration and bactericidal action include:
- Macrolides (e.g., Azithromycin): Not directly bactericidal but impair quorum sensing and biofilm integrity.
- DNase (e.g., Dornase alfa): Reduces mucus viscosity and improves drug diffusion.
- Adjunctive molecules: Compounds like gallium disrupt iron metabolism, weakening biofilms and enhancing antibiotic effects.
Clinical Evidence Supporting Combination Therapy in CF
Multiple clinical trials and cohort studies support the efficacy of synergistic antibiotic use:
- The ELITE study demonstrated improved lung function with alternating aztreonam and tobramycin inhalation.
- A Cochrane review noted improved short-term outcomes with dual IV antibiotics during exacerbations.
- Longitudinal CF registries show reduced exacerbation rates and hospitalizations with maintenance inhaled combination therapy.
Implementation of Synergy in Clinical Practice
To ensure therapeutic success, synergy must be integrated into patient-specific treatment plans. This involves:
- Sputum culture and sensitivity analysis to guide combination selection
- Serial pulmonary function testing to monitor response
- Pharmacokinetic/pharmacodynamic (PK/PD) optimization based on lung tissue penetration
- Alternating or cyclic therapy to minimize resistance and maintain efficacy
Collaborative management among pulmonologists, infectious disease specialists, and pharmacists is essential for tailoring regimens.
Synergistic Therapy as a Cornerstone of CF Care
Managing Pseudomonas aeruginosa infections in cystic fibrosis requires a proactive and dynamic therapeutic approach. Synergistic antibiotic regimens, particularly those incorporating inhaled formulations, systemic combinations, and biofilm-disrupting agents, represent the gold standard for reducing bacterial burden and preserving lung function. As resistance patterns evolve, ongoing research and clinical vigilance remain critical to sustaining treatment efficacy in this vulnerable population.