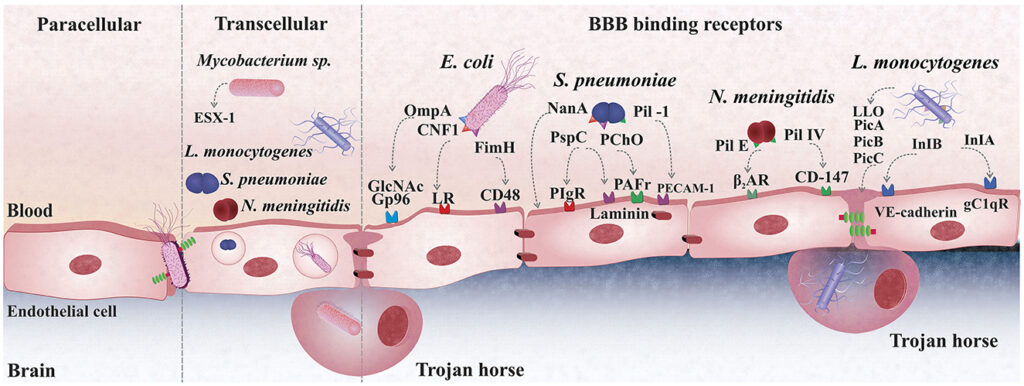
Escherichia coli (E. coli), a Gram-negative bacillus, is one of the most common etiologic agents of bacterial meningitis, especially in neonates and immunocompromised individuals. Among its pathogenic strains, those possessing the K1 capsular antigen demonstrate a high level of virulence and central nervous system (CNS) invasion. The increasing prevalence of extended-spectrum beta-lactamase (ESBL)-producing and carbapenem-resistant E. coli strains has heightened the urgency of optimizing treatment strategies through synergistic antibiotic therapy.
Mechanisms of Pathogenesis in E. coli Meningitis
E. coli penetrates the blood-brain barrier (BBB) through a combination of bacterial adhesion molecules, immune evasion strategies, and inflammatory cascades. The K1 capsule inhibits phagocytosis, while outer membrane proteins facilitate endothelial attachment. Once in the cerebrospinal fluid (CSF), bacterial replication induces cytokine storms and leukocyte infiltration, contributing to neuronal damage.
Limitations of Monotherapy in E. coli Meningitis
Standard empiric monotherapy, such as third-generation cephalosporins (e.g., cefotaxime, ceftriaxone), is often insufficient in severe or drug-resistant infections. These limitations include:
- Inadequate bactericidal activity against ESBL- and AmpC-producing strains
- Suboptimal CSF penetration of some agents
- Potential for resistance selection during therapy
To address these limitations, synergistic antibiotic regimens are increasingly recommended to enhance bactericidal activity, prevent resistance, and ensure deeper CNS tissue penetration.
Synergistic Antibiotic Strategies Against E. coli in Meningitis
1. Third-Generation Cephalosporins + Aminoglycosides
Example: Cefotaxime + Gentamicin
Mechanism:
- Cefotaxime disrupts bacterial cell wall synthesis.
- Gentamicin enters through compromised membranes to inhibit protein synthesis.
Benefits:
- Proven synergy in vitro and in vivo
- Rapid bactericidal activity in CSF
- Especially effective in neonatal meningitis
Caution:
- Risk of nephrotoxicity and ototoxicity with prolonged aminoglycoside use
- Gentamicin CSF penetration is low; synergy dependent on cefotaxime
2. Carbapenems + Polymyxins
Indication: ESBL or Carbapenem-resistant E. coli
Example: Meropenem + Colistin
Mechanism:
- Meropenem inhibits cell wall synthesis.
- Colistin disrupts outer membrane integrity and increases permeability.
Benefits:
- Useful in multidrug-resistant (MDR) or extensively drug-resistant (XDR) strains
- Colistin enhances intracellular uptake of carbapenems
- Effective salvage therapy for resistant CNS infections
3. Beta-Lactam + Beta-Lactamase Inhibitor Combinations
Example: Ceftazidime-avibactam
Indication: KPC-producing or ESBL-expressing E. coli
Advantages:
- Broad-spectrum coverage
- CSF penetration shown in pharmacokinetic studies
- Avibactam restores activity of ceftazidime against resistant strains
Synergy Evidence:
- In vitro models support enhanced bacterial kill
- Use in combination with fosfomycin or aminoglycosides may further potentiate efficacy
4. Fosfomycin-Based Synergistic Therapy
Example: Fosfomycin + Meropenem or Ceftazidime
Mechanism:
- Fosfomycin inhibits early-stage cell wall synthesis (MurA inhibition)
- Acts synergistically with beta-lactams due to complementary action on peptidoglycan pathway
Applications:
- MDR and CNS-penetrating combinations
- Emerging role in neonatal and pediatric resistant cases
5. Tigecycline and Adjunctive Agents
Note: Tigecycline has poor CSF penetration alone, but synergy with colistin and carbapenems has shown promise in intracranial infections caused by XDR E. coli. However, data remain limited and largely restricted to salvage scenarios.
Clinical Recommendations Based on Resistance Profiles
Susceptible E. coli (Non-ESBL, K1-negative)
- Preferred Regimen: Cefotaxime or Ceftriaxone monotherapy
- Alternative (severe cases): Cefotaxime + Gentamicin for initial 72 hours
ESBL-Producing E. coli
- Preferred Regimen: Meropenem
- With synergy: Meropenem + Fosfomycin or Meropenem + Colistin
Carbapenem-Resistant E. coli
- Options:
- Ceftazidime-avibactam + Aztreonam
- Meropenem + Colistin
- Fosfomycin + Tigecycline (salvage use)
Neonatal E. coli Meningitis
- Initial Empiric Therapy: Ampicillin + Cefotaxime
- Confirmed ESBL: Shift to Meropenem ± Gentamicin
Pharmacokinetics and CSF Penetration
CSF penetration is critical in meningitis management. Factors affecting drug levels include meningeal inflammation, molecular size, lipophilicity, and protein binding.
| Antibiotic | CSF Penetration (%) | Remarks |
|---|---|---|
| Cefotaxime | 10–40% | Reliable in inflamed meninges |
| Meropenem | 10–20% | Effective with high dosing |
| Colistin (IV) | <10% | Often combined with intrathecal administration |
| Gentamicin | <5% | Relies on synergy; poor monotherapy choice |
| Fosfomycin | 30–50% | Excellent for synergy |
| Ceftazidime-avibactam | ~10–15% | Effective in inflamed meninges |
Monitoring and Toxicity Considerations
- Gentamicin: Monitor peak/trough, renal function
- Colistin: Nephrotoxicity risk, neurotoxicity
- Carbapenems: Risk of seizures in high doses, especially imipenem
- Fosfomycin: Generally well-tolerated
- Tigecycline: Nausea, hepatotoxicity, poor CNS levels
Adjunctive Strategies for Better Outcomes
- Intrathecal or Intraventricular Antibiotics: Reserved for recalcitrant CNS infections, especially when systemic therapy fails
- Steroids (e.g., dexamethasone): Use cautiously; may reduce inflammatory damage but also reduce antibiotic penetration
- Source control: Timely CSF drainage in hydrocephalus or empyema
Synergistic antibiotic therapy is central to managing Escherichia coli meningitis, particularly in the context of rising antimicrobial resistance. Combinations such as cefotaxime-gentamicin for susceptible strains and carbapenem-colistin or beta-lactam-fosfomycin regimens for resistant strains provide superior bactericidal efficacy and improved outcomes. Tailoring therapy based on resistance patterns, patient factors, and CSF pharmacokinetics is essential for maximizing success in this high-stakes neurological infection.