Enterobacter species, primarily Enterobacter cloacae and Enterobacter aerogenes (now Klebsiella aerogenes), are gram-negative, facultative anaerobic bacilli within the Enterobacteriaceae family. While commonly colonizing the gastrointestinal tract, these organisms are opportunistic pathogens in hospitalized and immunocompromised patients. Their role in skin and skin structure infections (SSSIs) has grown due to increasing antimicrobial resistance and prevalence in surgical site and wound infections.
Pathogenesis and Virulence Mechanisms of Enterobacter
The pathogenicity of Enterobacter in soft tissue infections stems from its:
- Beta-lactamase production: Including AmpC and ESBLs, conferring resistance to many cephalosporins
- Biofilm formation: Promotes survival on wound surfaces and medical devices
- Adhesins and invasins: Enhance tissue colonization and immune evasion
- Endotoxins: Trigger host inflammatory responses and systemic symptoms
These features allow Enterobacter to establish persistent infections in compromised skin environments, particularly where wound healing is delayed.
Epidemiology of Skin and Skin Structure Enterobacter Infections
Enterobacter is commonly isolated in:
- Surgical site infections (abdominal, thoracic, orthopedic)
- Burn wounds
- Chronic ulcers and diabetic foot infections
- Traumatic wounds contaminated by fecal flora
- Post-catheterization or pressure injuries in long-term care facilities
Nosocomial acquisition is frequent, and outbreaks are associated with contamination of devices, antiseptics, or improper sterilization.
Clinical Manifestations of Enterobacter SSTIs
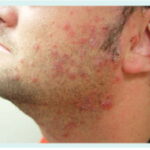
1. Cellulitis and Erysipelas-like Infections
- Rapidly spreading erythema, warmth, and pain
- Fever, leukocytosis
- Common in lower limbs, often misdiagnosed as streptococcal cellulitis
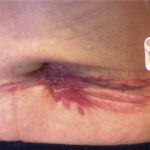
2. Wound Infections
- Purulent discharge, delayed healing
- Foul-smelling exudate in necrotic wounds
- Frequently polymicrobial in chronic wounds
3. Abscesses and Soft Tissue Collections
- Localized, fluctuant swelling
- Ultrasound often needed for diagnosis
- Incision and drainage required before antibiotic therapy
4. Necrotizing Infections
- Rare but severe presentations
- Extensive tissue destruction, systemic toxicity
- Often involves multiple drug-resistant organisms, including Enterobacter
Risk Factors and Populations at Risk
| Risk Factor | Mechanism of Susceptibility |
|---|---|
| Recent surgery or trauma | Direct tissue invasion |
| Diabetes mellitus | Impaired immunity and wound healing |
| Immunosuppression | Inability to control bacterial spread |
| Prolonged hospitalization | Exposure to nosocomial flora |
| Invasive devices (catheters, drains) | Colonization and biofilm formation |
| Broad-spectrum antibiotic use | Selection of resistant Enterobacter strains |
Diagnostic Evaluation and Laboratory Workup
Clinical Assessment
- Visual inspection of the wound and surrounding tissues
- Documentation of local and systemic signs of infection
- Evaluation of comorbid conditions and potential sources
Microbiological Testing
- Wound cultures: Quantitative or semi-quantitative; avoid surface swabs
- Blood cultures: Mandatory in febrile or systemically ill patients
- Antibiotic susceptibility testing: Identifies resistance to beta-lactams, carbapenems, quinolones
- Gram stain: Reveals gram-negative rods, supporting early treatment decisions
Imaging
- Ultrasound or CT: To assess abscesses or deep tissue involvement
- MRI: Preferred in suspected necrotizing fasciitis or osteomyelitis
Antibiotic Therapy and Resistance Considerations
Empiric Antibiotic Choices
Initial treatment must cover potential multidrug resistance:
- Carbapenems (meropenem, imipenem): Especially for ESBL-producing strains
- Piperacillin-tazobactam: For moderate infections where resistance is less likely
- Aminoglycosides (amikacin, gentamicin): Often added for synergy
- Fluoroquinolones: Only if susceptibility is confirmed
Directed Therapy
Tailored after culture results:
- Cefepime or levofloxacin: For susceptible, non-ESBL-producing isolates
- Avoid third-generation cephalosporins in AmpC producers (risk of resistance development during therapy)
- Duration:
- Uncomplicated cellulitis: 7–10 days
- Deep tissue or surgical site infections: 14–21 days
- Necrotizing infections: individualized, often prolonged
Surgical and Supportive Interventions
- Debridement: Critical for infection control and tissue regeneration
- Drainage: Necessary for abscesses and fluid collections
- Negative-pressure wound therapy (NPWT): Enhances healing, reduces bacterial load
- Glycemic and nutritional optimization: Supports immune response and tissue repair
- Pain control and wound care: Integral to patient recovery and compliance
Infection Control and Prevention Strategies
- Contact precautions in hospitalized or colonized patients
- Aseptic technique in wound dressing and surgical procedures
- Environmental disinfection to prevent nosocomial outbreaks
- Surveillance cultures during outbreaks to identify carriers
- Antibiotic stewardship to limit resistance pressure
Complications of Untreated Enterobacter Skin Infections
| Complication | Description |
|---|---|
| Bacteremia | Spread from infected wound to bloodstream |
| Sepsis | Systemic inflammatory response, may lead to shock |
| Chronic wound infection | Persistent colonization delays healing |
| Tissue necrosis | Requires surgical excision or amputation |
| Osteomyelitis | From contiguous spread, especially in diabetics |
Prompt, aggressive management prevents progression and improves prognosis.
Prognosis and Outcomes
Clinical outcome depends on infection severity, comorbidities, and timely initiation of effective therapy. Carbapenem-resistant Enterobacter infections carry higher morbidity.
| Infection Type | Prognosis | Mortality Rate |
|---|---|---|
| Mild cellulitis | Excellent with early antibiotics | <5% |
| Post-surgical infection | Variable, based on wound care | 10–20% |
| Necrotizing fasciitis | Guarded, requires ICU support | 30–50% |
Enterobacter species have become increasingly important in the landscape of skin and skin structure infections, especially in the hospital setting and among vulnerable patients. Their inherent and acquired antibiotic resistance mechanisms demand a focused diagnostic and therapeutic approach. Optimal outcomes require early recognition, appropriate antimicrobial selection, and, when necessary, surgical intervention. Effective infection control and preventive strategies further reduce transmission risks and healthcare burdens.