RET (REarranged during Transfection) gene fusions are oncogenic drivers identified across various solid tumor types. These fusions result in a chimeric oncoprotein combining the RET tyrosine kinase domain with a partner gene, promoting constitutive kinase activation and unregulated cell growth.
RET fusions have been detected in:
- Non-small cell lung cancer (NSCLC): 1–2% of cases
- Papillary thyroid carcinoma (PTC): 10–20% of sporadic cases, >60% of radiation-induced cases
- Colorectal, pancreatic, and salivary gland cancers: rare but clinically significant
- Breast, ovarian, and soft tissue tumors: rare occurrences
RET fusion-positive tumors often exhibit aggressive behavior but are susceptible to precision-targeted therapies that significantly improve clinical outcomes.
Molecular Pathogenesis of RET Fusion-Positive Cancers
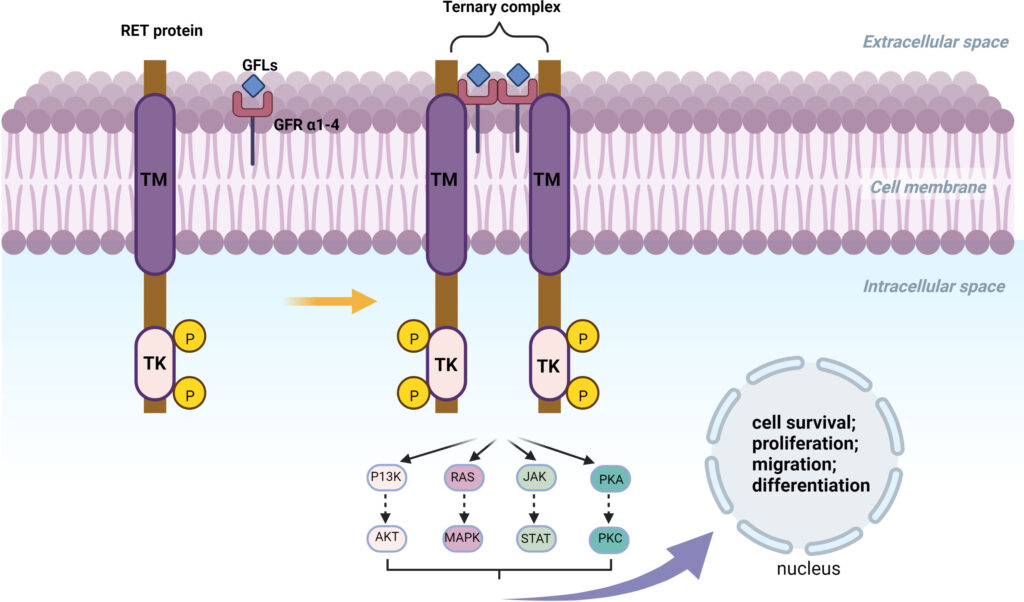
RET gene fusions occur when the kinase domain of RET fuses with various upstream genes such as:
- KIF5B-RET
- CCDC6-RET
- NCOA4-RET
These fusions lead to ligand-independent dimerization and downstream activation of proliferative pathways.
The presence of RET fusions transforms normal signaling into persistent oncogenic activation, making them ideal targets for therapy.
Clinical Spectrum of RET Fusion-Positive Solid Tumors
RET Fusions in Lung Cancer
- Present in 1–2% of NSCLC, particularly adenocarcinomas
- More common in younger, non-smoking patients
- Often associated with brain metastases and poor prognosis in the absence of targeted therapy
RET Fusions in Thyroid Cancer
- Especially prevalent in papillary thyroid carcinoma
- Strongly associated with radiation exposure and pediatric cases
- Frequently retain iodine sensitivity, but become refractory in advanced disease
Other Solid Tumors with RET Fusions
- Detected in a small subset of pancreatic, salivary gland, colorectal, and breast cancers
- Less common, but potentially responsive to RET-targeted therapy
- May present at advanced stages, often with metastatic disease
Diagnostic Methods for Detecting RET Fusions
Accurate diagnosis of RET fusion-positive tumors is critical for therapy selection. Recommended diagnostic modalities include:
Tissue-Based Molecular Testing
- Next-Generation Sequencing (NGS): Preferred method for comprehensive fusion detection
- Fluorescence In Situ Hybridization (FISH): Detects RET rearrangements but lacks partner identification
- Reverse Transcription PCR (RT-PCR): Sensitive to known fusion partners only
Liquid Biopsy
- Utilized when tissue is limited or inaccessible
- Identifies circulating tumor DNA (ctDNA) containing RET rearrangements
- Increasingly used in real-time monitoring of response and resistance
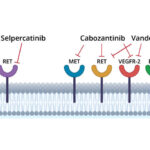
Targeted Therapies for RET Fusion-Positive Solid Tumors
Selective RET inhibitors have revolutionized the management of RET fusion-positive cancers. These agents show superior efficacy with fewer off-target effects compared to multikinase inhibitors.
Selpercatinib
- First FDA-approved RET-selective kinase inhibitor
- Indicated for RET fusion-positive NSCLC, thyroid cancers, and other solid tumors
- Demonstrated ORR > 70% in NSCLC and thyroid cancer
- Effective against CNS metastases due to blood-brain barrier penetration
- Adverse effects: hypertension, liver enzyme elevation, dry mouth
Pralsetinib
- Approved for RET fusion-positive NSCLC and thyroid cancer
- Similar efficacy to selpercatinib with robust intracranial activity
- Shows activity across a wide range of RET fusion partners
- Side effects: neutropenia, fatigue, increased ALT/AST
Both therapies offer durable disease control and are integral to the standard of care for advanced RET fusion-positive tumors.
Mechanisms of Resistance and Future Therapeutics
Despite initial responses, some patients develop acquired resistance to RET inhibitors:
- Solvent front mutations (e.g., RET G810R/S/C) hinder drug binding
- Bypass signaling via MET, EGFR, or KRAS activation
- Histologic transformation to more aggressive variants
Investigational Approaches
- Next-generation RET inhibitors targeting resistance mutations (e.g., TPX-0046)
- Combination therapy with MET inhibitors or MEK inhibitors
- Immunotherapy combinations in selected patients with high tumor mutational burden
Ongoing clinical trials aim to extend progression-free survival and address treatment-resistant disease.
Clinical Outcomes and Prognosis
Patients treated with selective RET inhibitors experience:
- Rapid and durable responses
- Median progression-free survival (PFS): ~18–20 months in NSCLC
- Overall survival (OS): improved, especially in treatment-naïve cohorts
- High intracranial disease control, critical for brain metastasis management
In previously RAI-refractory thyroid cancers, some patients demonstrate redifferentiation, enabling renewed RAI uptake.
Follow-Up and Disease Monitoring
Long-term management includes:
- Radiologic imaging (CT, MRI, PET-CT for metastasis evaluation)
- Biomarker monitoring (e.g., thyroglobulin in thyroid cancer)
- Repeat molecular profiling upon progression
- Surveillance for cardiovascular and hepatic adverse effects
Regular multidisciplinary evaluations are essential to optimize therapy and transition between treatment lines.
Frequently Asked Questions:
What is a RET fusion-positive tumor?
A tumor driven by the abnormal fusion of the RET gene with another gene, resulting in persistent oncogenic activity.
Which cancers can have RET fusions?
Most commonly found in non-small cell lung cancer and papillary thyroid cancer; also observed in colorectal, breast, and pancreatic cancers.
How are RET fusions detected?
Using NGS, FISH, or RT-PCR on tissue or liquid biopsies.
Are RET fusion-positive tumors treatable?
Yes, with FDA-approved selective RET inhibitors like selpercatinib and pralsetinib offering high response rates.
Can RET fusion-positive tumors become resistant to therapy?
Yes, but new drugs and combinations are being developed to overcome resistance mechanisms.