RET (Rearranged during Transfection) fusion-positive metastatic non-small cell lung cancer (NSCLC) represents a rare but clinically significant molecular subset of lung adenocarcinoma. RET gene fusions result from chromosomal rearrangements that juxtapose the RET tyrosine kinase domain to partner genes such as KIF5B, CCDC6, or NCOA4, leading to aberrant activation of oncogenic signaling pathways.
RET fusion is observed in approximately 1–2% of NSCLC cases, predominantly among younger, non-smoking patients with adenocarcinoma histology. Accurate molecular characterization is essential for targeted treatment.
Molecular Biology of RET Fusion-Positive NSCLC
RET fusions activate signaling pathways involved in cell growth, differentiation, and survival, including:
- MAPK/ERK
- PI3K/AKT
- JAK/STAT
These fusions are mutually exclusive with other driver mutations such as EGFR, ALK, or ROS1, underscoring the need for comprehensive next-generation sequencing (NGS) during diagnosis.
Clinical Presentation and Diagnosis
Patients with RET fusion-positive metastatic NSCLC commonly present with symptoms similar to other advanced lung cancers:
- Persistent cough
- Hemoptysis
- Dyspnea
- Unintentional weight loss
- Bone or brain metastases in advanced stages
Diagnosis is confirmed through:
- Tissue biopsy with histologic confirmation of adenocarcinoma
- Molecular testing, preferably via NGS or fluorescence in situ hybridization (FISH), to identify RET fusions
- Liquid biopsy (cfDNA) as an alternative when tissue is insufficient
Current Standard of Care and RET-Targeted Therapies
The development of selective RET inhibitors has revolutionized the treatment landscape for this molecular subtype. These agents offer superior efficacy and tolerability compared to traditional chemotherapy or non-selective kinase inhibitors.
Selpercatinib (LOXO-292)
- FDA-approved for RET fusion-positive NSCLC
- High objective response rate (ORR ~64%)
- Durable responses with a favorable safety profile
- Effective against central nervous system (CNS) metastases
Pralsetinib (BLU-667)
- Also FDA-approved for RET fusion-positive NSCLC
- ORR of ~61% in treatment-naïve and ~70% in pre-treated patients
- Demonstrates intracranial activity
- Common adverse effects: hypertension, elevated liver enzymes, constipation
Chemotherapy and Immunotherapy
In the absence of RET-targeted therapy access, standard platinum-doublet chemotherapy (e.g., cisplatin + pemetrexed) remains an option. However, immunotherapy (e.g., PD-1/PD-L1 inhibitors) is generally less effective in RET-positive NSCLC due to lower tumor mutational burden and minimal PD-L1 expression.
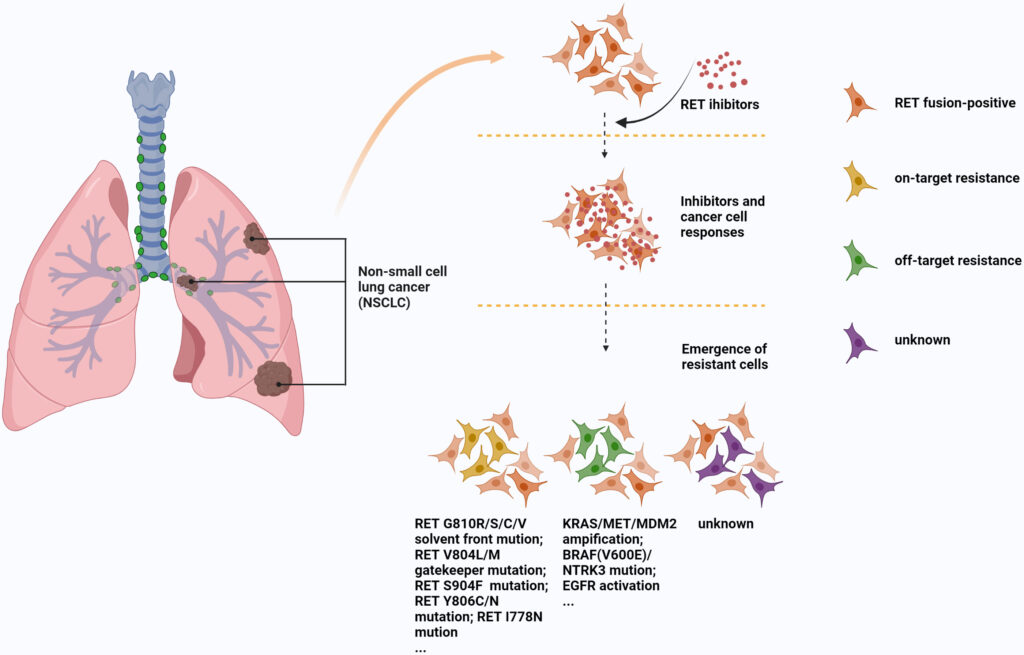
Mechanisms of Resistance to RET Inhibitors
Despite initial responses, some patients develop resistance to RET inhibitors. Mechanisms include:
- Secondary RET mutations (e.g., G810 solvent front mutations)
- Bypass signaling activation, such as MET amplification or KRAS mutation
- Histologic transformation or epithelial-mesenchymal transition (EMT)
Overcoming resistance may involve combination therapies or next-generation RET inhibitors currently under clinical investigation.
Prognosis and Survival Outlook
RET fusion-positive NSCLC, when treated with selective RET inhibitors, demonstrates promising outcomes:
- Median progression-free survival (PFS): ~17 months (selpercatinib)
- Median overall survival (OS): Not yet reached in early trials
- Favorable prognosis compared to non-targeted therapies, especially in patients with CNS metastases
Timely molecular diagnosis and access to RET-targeted therapy are critical for optimizing clinical outcomes.
Future Directions in RET-Positive Lung Cancer Research
Research is actively exploring:
- Combination regimens (RET inhibitors + MET or EGFR inhibitors)
- Next-generation RET inhibitors with enhanced activity against resistance mutations
- Biomarker-driven trials for patient stratification
- Immunotherapy combinations in selected patients with higher TMB or PD-L1 levels
Continued innovation will aim to delay resistance, enhance CNS control, and improve survival metrics.
Frequently Asked Questions:
What is RET fusion and how is it detected?
RET fusion is a chromosomal rearrangement causing oncogenic RET kinase activity. It is detected via next-generation sequencing, FISH, or PCR.
Are RET inhibitors better than chemotherapy?
Yes. Selective RET inhibitors provide higher response rates, longer progression-free survival, and better tolerability compared to chemotherapy.
Can RET fusion occur with EGFR or ALK mutations?
No. RET fusion is mutually exclusive with EGFR, ALK, and other common driver mutations in NSCLC.
Do RET inhibitors work for brain metastases?
Yes. Both selpercatinib and pralsetinib demonstrate significant intracranial activity in RET fusion-positive NSCLC.
Is RET testing necessary for all lung cancer patients?
RET testing is recommended for all advanced non-squamous NSCLC cases, especially in non-smokers and younger patients.