Refractory Mycobacterium avium complex (MAC) pulmonary disease occurs when the infection persists or progresses despite appropriate guideline-based therapy for at least 6 months. As part of the broader group of nontuberculous mycobacterial (NTM) infections, MAC pulmonary disease poses significant clinical challenges due to antibiotic resistance, host factors, and complex pathophysiology. Timely recognition and aggressive management are essential to improve outcomes.
Pathophysiology and Risk Factors
Pathophysiological Mechanisms
Refractory MAC pulmonary disease is driven by a combination of microbial and host-related factors:
- Biofilm Formation: Mycobacteria produce biofilms that protect them from host defenses and antibiotics.
- Antibiotic Resistance: Mutations in 23S rRNA and efflux pump overexpression reduce macrolide and aminoglycoside efficacy.
- Host Immune Dysfunction: Defects in IFN-γ and IL-12 pathways compromise the immune response.
Predisposing Conditions
Certain individuals are at higher risk of developing refractory disease:
- Structural lung disease (bronchiectasis, COPD, cystic fibrosis)
- Immunosuppression (HIV/AIDS, post-transplant state)
- Genetic factors (CFTR mutations, primary ciliary dyskinesia)
- History of gastroesophageal reflux disease (GERD)
Clinical Presentation and Diagnostic Approach
Symptoms of Refractory MAC Pulmonary Disease
Patients typically present with:
- Chronic cough with or without sputum production
- Fatigue and weight loss
- Hemoptysis
- Progressive dyspnea
- Low-grade fevers and night sweats
Symptoms tend to be more severe and persistent compared to initial disease.
Diagnostic Criteria
Diagnosis of refractory MAC pulmonary disease involves confirming ongoing active infection:
- Microbiological Confirmation: Positive cultures from at least two separate expectorated sputum samples, or one bronchoscopic specimen.
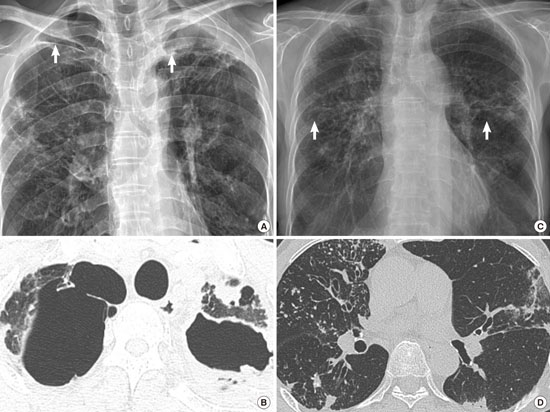
- Radiological Evidence: High-resolution CT (HRCT) findings of nodular-bronchiectatic changes or cavitary lesions.
- Clinical Symptoms: Persistence or progression despite appropriate therapy.
Therapeutic Strategies for Refractory MAC Pulmonary Disease
Optimization of Antimicrobial Therapy
Refractory disease demands modification and intensification of the antibiotic regimen:
- Macrolide-Based Backbone: Azithromycin or clarithromycin remains central, unless resistance develops.
- Addition of Injectable Agents: Amikacin or streptomycin added for at least the initial phase.
- Use of Clofazimine: As part of a multidrug regimen, clofazimine demonstrates activity against resistant MAC.
- Liposomal Amikacin Inhalation Suspension (ALIS): FDA-approved for refractory MAC to deliver high concentrations directly to the lungs.
Surgical Resection
For localized cavitary disease unresponsive to medical therapy, surgical resection of affected lung segments may be considered. Ideal candidates have:
- Limited pulmonary reserve impairment
- Disease confined to a lobe or lung segment
- Failure of optimized medical therapy
Adjunctive Therapies
- Airway Clearance Techniques: Physiotherapy to facilitate sputum expectoration and reduce bacterial load.
- Nutritional Support: Addressing weight loss and malnutrition to enhance host immunity.
- Management of Comorbidities: Control of GERD, diabetes, or other contributing conditions.
Antimicrobial Resistance and Its Implications
Resistance development, particularly to macrolides, is the hallmark of refractory disease and portends a poor prognosis. Mechanisms include:
- Mutation in rrl Gene: High-level resistance to clarithromycin.
- Efflux Pump Overactivity: Decreased intracellular drug concentrations.
In cases of macrolide-resistant MAC, alternative regimens incorporating rifamycins, ethambutol, clofazimine, and amikacin are used, often with diminished efficacy.
Prognosis and Risk Stratification
Prognostic Factors
Several factors influence treatment outcomes:
- Presence of cavitary disease
- Degree of macrolide resistance
- Host immune status
- Adherence to therapy
- Radiologic progression
Outcome Expectations
The overall success rate in refractory cases remains modest, with culture conversion rates ranging from 30% to 60% despite intensified therapy.
Future Perspectives in Management
Research continues to address unmet needs in refractory MAC pulmonary disease:
- New Antimicrobial Agents: Oxazolidinones, bedaquiline, and novel macrolides under evaluation.
- Host-Directed Therapies: Immune modulators aiming to bolster host defenses.
- Vaccination Strategies: Development of preventive vaccines against NTM infections.
- Personalized Medicine: Genomic profiling to predict treatment responses and tailor therapies.
Refractory Mycobacterium avium complex pulmonary disease represents a formidable clinical challenge requiring a personalized, multidisciplinary approach. Optimizing antimicrobial therapy, addressing host factors, considering surgical intervention, and incorporating emerging therapies offer the best strategies for managing this persistent infection. Continued research and innovation remain essential to improving patient outcomes in this difficult-to-treat disease.