Primary dysbetalipoproteinemia, also known as type III hyperlipoproteinemia, is a rare genetic lipid metabolism disorder characterized by the improper clearance of remnant lipoproteins from the bloodstream. This leads to elevated plasma cholesterol and triglyceride levels, increasing the risk of premature atherosclerosis and cardiovascular diseases. The condition is closely associated with mutations in the apolipoprotein E (ApoE) gene, specifically the ApoE2/E2 genotype.
Pathophysiology and Genetic Basis
Impaired Remnant Lipoprotein Clearance
Under normal physiological conditions, ApoE plays a pivotal role in mediating the hepatic uptake of chylomicron remnants and intermediate-density lipoproteins (IDLs). In primary dysbetalipoproteinemia, the ApoE2 isoform exhibits reduced binding affinity to hepatic lipoprotein receptors, resulting in accumulation of cholesterol-rich remnant particles.
ApoE Genotype Correlation
- ApoE2/E2: ~90% of affected individuals carry this genotype.
- Homozygosity alone is insufficient for clinical manifestation; secondary factors such as obesity, diabetes mellitus, hypothyroidism, or renal disease often trigger the disorder.
Clinical Manifestations
Hallmark Symptoms
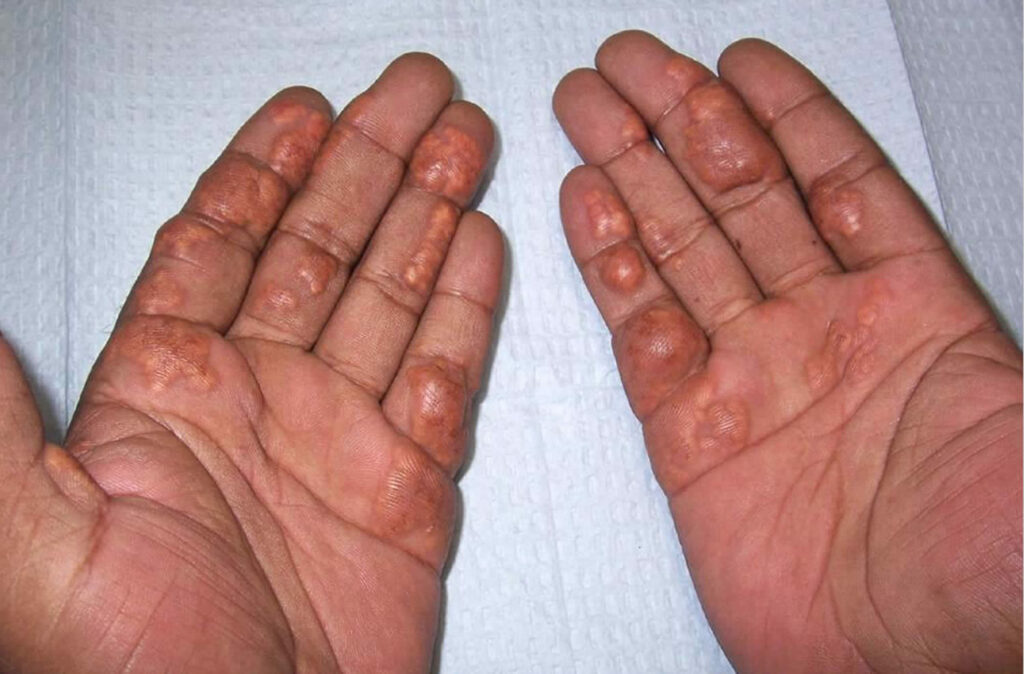
- Palmar xanthomas: Yellow-orange plaques on palms, considered pathognomonic.
- Tuberous xanthomas: Firm nodules on elbows and knees.
- Premature atherosclerosis: Often manifests as coronary artery disease or peripheral vascular disease in early adulthood.
Biochemical Profile
- Total cholesterol: Elevated (typically 300–600 mg/dL)
- Triglycerides: Elevated (250–500 mg/dL)
- VLDL remnants and IDL: Accumulated
- LDL: Often normal or low
- HDL: Usually decreased
Lipoprotein electrophoresis or ultracentrifugation reveals a broad beta band, indicative of remnant lipoprotein presence.
Diagnostic Approach
Lipid Panel Evaluation
Initial diagnosis is often suspected with elevated total cholesterol and triglycerides in roughly equal proportions. The cholesterol/triglyceride ratio remains near 1.0.
Confirmatory Testing
- ApoE genotyping: Identifies E2/E2 homozygosity.
- Lipoprotein subfraction analysis: Confirms presence of β-VLDL.
- Secondary cause evaluation: Rule out hypothyroidism, diabetes, or renal insufficiency.
Treatment Strategies
Lifestyle and Dietary Modifications
- Low-fat, low-cholesterol diet: Emphasize reduction of saturated fats and refined carbohydrates.
- Weight loss: Significantly improves lipid profile.
- Control of secondary triggers: Optimal management of diabetes, hypothyroidism, and obesity is essential.
Pharmacological Therapy
- Fibrates (e.g., gemfibrozil, fenofibrate): First-line agents targeting triglyceride reduction and remnant clearance.
- Statins: Lower LDL-C and have mild effects on triglycerides.
- Niacin: Reduces hepatic VLDL production.
- Omega-3 fatty acids: Adjunctive therapy for hypertriglyceridemia.
Combination therapy is often warranted to achieve target lipid levels and reduce cardiovascular risk.
Long-Term Management and Prognosis
Cardiovascular Risk Reduction
Due to heightened risk of atherosclerosis, aggressive management of lipid abnormalities and modifiable risk factors is crucial. Lifelong follow-up with a lipid specialist is often recommended.
Screening Recommendations
- First-degree relatives of affected individuals should undergo lipid screening and ApoE genotyping.
- Individuals with unexplained mixed hyperlipidemia and xanthomas should be evaluated for type III hyperlipoproteinemia.
Primary Dysbetalipoproteinemia vs Other Lipid Disorders
| Feature | Type IIa (Familial Hypercholesterolemia) | Type IV (Hypertriglyceridemia) | Type III (Dysbetalipoproteinemia) |
|---|---|---|---|
| ApoE mutation | No | No | Yes (E2/E2) |
| Cholesterol | Elevated | Normal/Moderately elevated | Elevated |
| Triglycerides | Normal | Elevated | Elevated |
| Xanthomas | Tendinous | Rare | Palmar/Tuberous |
| Cardiovascular Risk | High | Moderate | High |
Primary dysbetalipoproteinemia represents a unique lipid disorder driven by genetic and metabolic factors, with significant implications for cardiovascular health. Early diagnosis through clinical, biochemical, and genetic evaluation enables timely intervention, reducing the risk of premature vascular disease. With personalized treatment strategies, most patients can achieve effective lipid control and improved long-term outcomes.