Liver transplantation stands as a critical intervention for patients with end-stage liver disease. Despite advancements in surgical techniques and immunosuppressive therapies, liver transplant rejection remains a pivotal concern. The effective prevention of liver transplant rejection hinges on comprehensive immunologic evaluation, strategic immunosuppression, and continuous post-transplant surveillance.
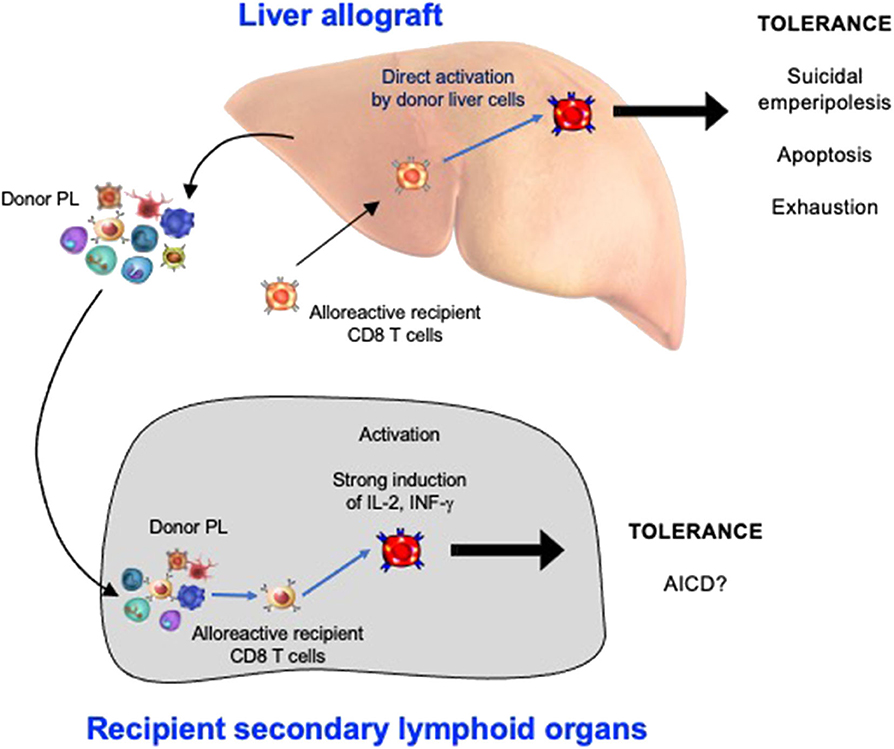
Understanding Liver Transplant Rejection
Rejection occurs when the recipient’s immune system identifies the transplanted liver as a foreign object and initiates an immune response. Liver allograft rejection is classified into distinct categories:
1. Hyperacute Rejection
- Onset: Minutes to hours post-transplant
- Cause: Preformed donor-specific antibodies (DSAs)
- Prevention: ABO compatibility and crossmatch testing
2. Acute Cellular Rejection (ACR)
- Onset: Days to weeks post-transplant
- Cause: T-cell mediated immune response
- Features: Portal inflammation, bile duct damage, endotheliitis
- Diagnosis: Liver biopsy with Banff criteria scoring
3. Chronic Rejection
- Onset: Weeks to months, gradual onset
- Cause: Inadequate immunosuppression or recurrent ACR
- Pathology: Loss of bile ducts (ductopenia), vanishing bile duct syndrome
- Outcome: Irreversible, often leads to re-transplantation
Immunosuppressive Therapy Protocols in Liver Transplantation
Effective immunosuppressive management is central to preventing graft rejection while avoiding adverse effects such as infections and malignancies.
1. Induction Therapy
Administered during the perioperative period to reduce early immune activation.
- Agents Used:
- Basiliximab (IL-2 receptor antagonist)
- Methylprednisolone (high-dose steroid pulses)
2. Maintenance Immunosuppression
Long-term regimen designed to suppress T-cell activity and antibody production.
| Class | Examples | Mechanism of Action |
|---|---|---|
| Calcineurin Inhibitors | Tacrolimus, Cyclosporine | Inhibit IL-2 production and T-cell response |
| Antimetabolites | Mycophenolate mofetil, Azathioprine | Inhibit DNA synthesis in lymphocytes |
| Corticosteroids | Prednisone | Anti-inflammatory and immunosuppressive |
| mTOR Inhibitors | Sirolimus, Everolimus | Block cell proliferation via mTOR pathway |
Tacrolimus is the gold standard for maintenance due to its lower rejection rates and improved graft survival metrics.
Personalized Immunosuppressive Strategies Based on Recipient Risk
Recipient-specific factors necessitate tailored immunosuppressive regimens:
High-Risk Patients
- Pediatric recipients
- African ancestry
- Re-transplantation cases
- HLA mismatches
- Presence of pre-transplant DSAs
These patients may require dual therapy or higher-dose tacrolimus with close therapeutic drug monitoring (TDM).
Therapeutic Drug Monitoring and Dose Adjustment
Maintaining immunosuppressive drug levels within target ranges is critical to prevent both under- and over-immunosuppression.
| Drug | Target Trough Level (ng/mL) | Time Post-Transplant |
|---|---|---|
| Tacrolimus | 8–12 | First month |
| Tacrolimus | 5–8 | After 3 months |
| Cyclosporine | 100–250 | Varies by protocol |
Frequent adjustments are required in cases of:
- Renal dysfunction
- Hepatic impairment
- Drug-drug interactions (e.g., antifungals, antibiotics)
Monitoring and Diagnostic Tools for Early Rejection Detection
1. Biochemical Monitoring
- Serum ALT, AST, Bilirubin, ALP, GGT: Sudden elevations suggest hepatocellular injury or cholestasis
- INR and Albumin: Functional assessment of liver synthesis
2. Liver Biopsy
Gold standard for diagnosing acute and chronic rejection. Utilizes Banff Rejection Activity Index (RAI) to grade severity.
3. Non-Invasive Biomarkers and Imaging
- Donor-derived cell-free DNA (dd-cfDNA)
- Liver stiffness measurement using transient elastography (FibroScan)
- Immunologic assays detecting circulating DSAs
Managing Infections Without Compromising Rejection Prevention
Balancing infection prophylaxis with immune suppression is crucial.
Common Post-Transplant Infections
- Cytomegalovirus (CMV)
- Epstein-Barr Virus (EBV)
- Fungal infections (Candida, Aspergillus)
Prophylactic Regimens
- Valganciclovir for CMV
- Trimethoprim-sulfamethoxazole for Pneumocystis pneumonia
- Fluconazole for fungal coverage
Adjustment of immunosuppression during infection is performed cautiously to avoid precipitating acute rejection.
Medication Adherence: The Keystone of Rejection Prevention
Non-compliance with immunosuppressive therapy remains a leading cause of late rejection.
Adherence Promotion Strategies
- Education programs focused on lifelong therapy commitment
- Digital adherence tools including pill trackers and mobile reminders
- Psychological support and routine counseling
- Family involvement in post-transplant care
Long-Term Graft Surveillance and Lifestyle Interventions
Chronic liver graft survival is enhanced through continuous evaluation and management of comorbidities.
Key Interventions
- Control of metabolic syndrome: Weight management, statins, glycemic control
- Avoidance of hepatotoxic substances: Alcohol, herbal supplements
- Cancer surveillance: Skin exams, colorectal and liver cancer screening
- Bone health: Vitamin D and calcium supplementation to counter steroid-induced osteoporosis
Frequently Asked Questions:
What is the first sign of liver transplant rejection?
An elevation in liver enzymes (ALT, AST) often precedes clinical symptoms and signals early graft injury.
Can liver transplant rejection be cured?
Acute rejection is often reversible with timely adjustment of immunosuppressive therapy. Chronic rejection, however, may lead to graft failure.
How long does immunosuppressive therapy last?
Lifelong immunosuppression is required to prevent both early and late allograft rejection.
What are the risks of over-immunosuppression?
Over-suppression increases susceptibility to life-threatening infections, malignancies, and metabolic complications.
Can rejection occur even after many years?
Yes. Late-onset rejection can occur due to non-adherence, infections, or reduction in immunosuppression.
The prevention of liver transplant rejection is a cornerstone of long-term transplant success. By integrating individualized immunosuppressive protocols, rigorous monitoring, and proactive lifestyle management, we can significantly enhance graft survival and patient quality of life. Continued innovation in biomarkers and non-invasive diagnostics further refines our ability to intervene early, ensuring optimal outcomes for liver transplant recipients.