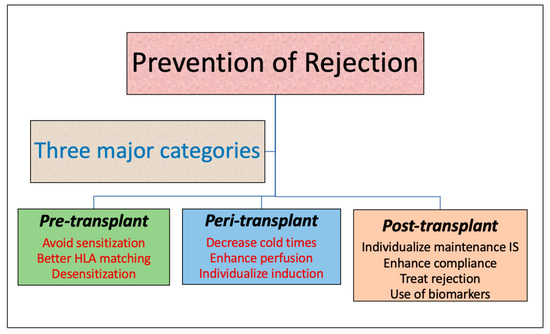
Kidney transplantation offers life-saving treatment for patients with end-stage renal disease. However, graft rejection remains a primary challenge impacting long-term outcomes. Preventing kidney transplant rejection requires a multifaceted, evidence-driven approach encompassing tailored immunosuppressive regimens, vigilant monitoring, and timely intervention.
Understanding Kidney Transplant Rejection
Rejection occurs when the recipient’s immune system identifies the transplanted kidney as foreign and mounts an immune response. It is classified into three primary types:
1. Hyperacute Rejection
- Occurs minutes to hours post-transplant
- Caused by preformed antibodies (e.g., anti-HLA or ABO)
- Prevention: Crossmatch testing and ABO compatibility screening
2. Acute Rejection
- Appears within weeks to months
- May be cellular (T-cell mediated) or humoral (antibody-mediated)
- Prevention relies on immunosuppression and regular surveillance
3. Chronic Rejection
- Develops over months to years
- Characterized by interstitial fibrosis, tubular atrophy, and vasculopathy
- No definitive cure; focus is on prevention and slowing progression
Essential Immunosuppressive Protocols for Rejection Prevention
1. Induction Therapy (Immediate post-transplant phase)
Used to minimize the initial immune response, especially in high-risk recipients.
- Monoclonal or polyclonal antibodies:
- Basiliximab (IL-2 receptor blocker)
- Antithymocyte globulin (ATG)
- Tailored based on panel reactive antibody (PRA) level, donor type, and immunologic risk
2. Maintenance Immunosuppression (Lifelong therapy)
A triple-drug regimen is commonly adopted:
| Drug Class | Examples | Mechanism |
|---|---|---|
| Calcineurin Inhibitors | Tacrolimus, Cyclosporine | Suppress T-cell activation |
| Antimetabolites | Mycophenolate mofetil (MMF), Azathioprine | Inhibit lymphocyte proliferation |
| Corticosteroids | Prednisone | Broad immunosuppressive effects |
Tacrolimus is the preferred calcineurin inhibitor due to lower acute rejection rates.
Monitoring and Early Detection of Rejection
Timely identification of rejection improves outcomes and preserves graft function.
1. Routine Clinical Monitoring
- Serum creatinine and eGFR: Elevations may indicate dysfunction
- Blood pressure and proteinuria: Markers of graft stress or injury
- Immunosuppressant drug levels: Trough levels must be maintained in therapeutic range
2. Immunologic Biomarkers
Emerging technologies aid in non-invasive early detection:
- Donor-derived cell-free DNA (dd-cfDNA)
- Urinary chemokines (CXCL9, CXCL10)
- Gene expression profiling (e.g., AlloMap Kidney)
3. Protocol and Indication Biopsies
- Protocol biopsies: Performed at set intervals (e.g., 3, 6, 12 months) even if patient is asymptomatic
- Indication biopsies: Triggered by graft dysfunction or clinical suspicion
Risk Factors and Personalized Immunosuppression
High-Risk Categories
- Young recipients
- African ancestry
- Repeat transplant recipients
- High PRA titers
- Delayed graft function
These groups require more potent induction therapy, closer surveillance, and possible adjustment in maintenance regimens.
Strategies to Enhance Medication Adherence
Non-adherence is a significant cause of late acute rejection and graft loss.
- Patient education about long-term risks of non-compliance
- Mobile health apps for medication reminders
- Frequent follow-ups to reinforce adherence
- Psychosocial support for high-risk individuals
Infection Prophylaxis and Rejection Balance
Over-immunosuppression increases infection risk, especially cytomegalovirus (CMV), BK virus, and opportunistic infections.
Preventive Measures:
- CMV prophylaxis: Valganciclovir in seropositive donors or high-risk recipients
- BK virus monitoring: PCR testing at regular intervals
- Adjust immunosuppressive therapy to balance infection and rejection risk
Long-Term Graft Survival: Optimizing Comorbid Conditions
1. Cardiovascular Risk Management
- Hypertension, dyslipidemia, and diabetes must be aggressively managed
- Preferred agents: Calcium channel blockers, statins, SGLT2 inhibitors
2. Cancer Surveillance
- Long-term immunosuppression increases skin cancer, lymphoma, and solid organ malignancy risks
- Annual dermatologic exams and appropriate cancer screenings are essential
3. Bone Health
- Steroids contribute to osteopenia and fractures
- Supplementation with vitamin D, calcium, and bisphosphonates when needed
Frequently Asked Questions:
What is the most common cause of kidney transplant rejection?
T-cell mediated acute rejection is the most prevalent type and typically occurs within the first six months if immunosuppression is inadequate.
How can rejection be detected early?
Through regular blood tests, biomarker monitoring, and protocol biopsies, early changes can be detected before irreversible damage occurs.
Can rejection be reversed?
Acute rejection, if caught early, can often be reversed with intensified immunosuppressive therapy. Chronic rejection is less responsive and focuses on preservation.
What lifestyle changes help prevent rejection?
Maintaining strict medication adherence, controlling blood pressure and glucose, avoiding infections, and regular follow-ups are critical for graft longevity.
Is immunosuppression lifelong?
Yes. Immunosuppressive therapy is necessary throughout the life of the graft to prevent both acute and chronic rejection.
The prevention of kidney transplant rejection is a dynamic process involving precise immunologic matching, tailored induction and maintenance therapy, rigorous monitoring, and patient-centered care. As we continue to advance in the fields of immunogenetics and transplant immunology, the focus remains steadfast on optimizing outcomes and prolonging graft survival through proactive, personalized strategies.