Graft-versus-host disease (GVHD) remains a significant complication following allogeneic hematopoietic stem cell transplantation (HSCT). Effective prevention is critical to improving transplant outcomes and minimizing morbidity and mortality. This article outlines current evidence-based strategies for GVHD prevention, including pharmacologic prophylaxis, donor selection, cellular manipulation, and risk-adapted approaches.
Understanding the Pathogenesis of GVHD
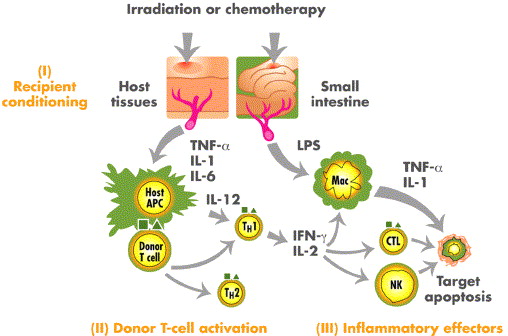
GVHD arises when immunocompetent donor T lymphocytes recognize recipient tissues as foreign and mount an immune response. The pathophysiology is a multistep process involving:
- Tissue Damage from Conditioning Regimen: Activates antigen-presenting cells (APCs)
- Donor T Cell Activation: Leads to proliferation and cytokine release
- Target Organ Damage: Primarily affects the skin, gastrointestinal tract, and liver
The prevention of GVHD hinges on interrupting this cascade, particularly by targeting T cell activation and inflammatory pathways.
Risk Factors and Stratification
Patient and Transplant-Related Risk Factors
- HLA mismatch between donor and recipient
- Unrelated or mismatched related donors
- Peripheral blood stem cell grafts vs. bone marrow
- Older age of recipient or donor
- Female donor to male recipient
- Myeloablative conditioning regimens
Risk stratification is essential for tailoring prophylactic regimens. High-risk patients may require intensified or dual-agent approaches.
Pharmacologic Prophylaxis: First-Line Approaches
Calcineurin Inhibitor-Based Regimens
Calcineurin inhibitors remain the cornerstone of GVHD prophylaxis.
Tacrolimus + Methotrexate
- Most widely used regimen in both matched sibling and unrelated donor transplants
- Methotrexate is typically administered on Days +1, +3, +6, and +11
- Tacrolimus is started pre-transplant (Day -1) and continued post-transplant with serum level monitoring
Cyclosporine + Methotrexate
- Preferred in some protocols; similar efficacy to tacrolimus
- Requires careful renal function monitoring
Mycophenolate Mofetil (MMF)
- Often used with calcineurin inhibitors in reduced-intensity conditioning or cord blood transplantation
- Advantages: faster engraftment, reduced mucositis
Advanced and Experimental Strategies
Post-Transplant Cyclophosphamide (PTCy)
- Common in haploidentical and mismatched unrelated donor settings
- Cyclophosphamide administered on Days +3 and +4 post-transplant
- Frequently combined with tacrolimus and MMF
- Reduces both acute and chronic GVHD incidence without delaying engraftment
Antithymocyte Globulin (ATG)
- Polyclonal antibody that depletes T cells
- Administered pre-transplant in unrelated donor settings
- Effective in reducing chronic GVHD but associated with delayed immune reconstitution and increased infection risk
T Cell Depletion Techniques
Ex Vivo T Cell Depletion
- Physically removes T cells from graft prior to infusion
- Reduces GVHD incidence but may impair graft-versus-leukemia (GVL) effect
In Vivo T Cell Depletion with Alemtuzumab
- Humanized anti-CD52 monoclonal antibody
- Used in reduced-intensity protocols to suppress early T cell activity
Cellular Therapy and Donor Selection
Donor Selection and HLA Matching
- HLA-identical sibling donors are ideal
- 10/10 allele-matched unrelated donors minimize GVHD risk
- Mismatched unrelated or haploidentical donors require intensified prophylaxis
Graft Source Considerations
| Graft Type | GVHD Risk | Notes |
|---|---|---|
| Bone Marrow | Lower | Preferred in pediatric patients |
| Peripheral Blood | Higher | Faster engraftment |
| Umbilical Cord Blood | Variable | Lower chronic GVHD |
Risk-Adapted and Personalized Prophylaxis
Emerging practices involve tailoring GVHD prophylaxis to patient-specific and transplant-related variables:
- High-risk patients (e.g., mismatched donors) receive PTCy or ATG
- Low-risk patients may benefit from reduced-intensity prophylaxis
- Ongoing clinical trials are evaluating biomarker-guided GVHD prevention, including:
- TNFR1, ST2, REG3α for acute GVHD prediction
Monitoring and Tapering of Immunosuppression
- Weekly monitoring of tacrolimus/cyclosporine levels
- Taper begins at 3–6 months post-transplant in the absence of GVHD
- Delayed taper or continuation advised in high-risk patients or those with prior GVHD
Prevention of Chronic GVHD
While acute GVHD occurs within 100 days post-transplant, chronic GVHD develops later and requires different preventive strategies:
- Use of bone marrow over peripheral blood to reduce incidence
- Prolonged immunosuppression in high-risk individuals
- Extracorporeal photopheresis and rituximab in select cases as prophylactic options
Frequently Asked Questions
What is the best prophylactic regimen for GVHD?
Tacrolimus plus methotrexate remains the gold standard for HLA-matched transplants. For haploidentical transplants, PTCy-based regimens are preferred.
Can GVHD be completely prevented?
Complete prevention is unlikely, but current strategies significantly reduce incidence and severity. Early detection and rapid intervention are critical.
How long is immunosuppression continued post-transplant?
Typically 3–6 months if no GVHD is observed. Longer durations are needed in patients with prior episodes or high-risk profiles.
Is T cell depletion used in all patients?
No. T cell depletion is reserved for high-risk settings due to potential compromise of the graft-versus-leukemia effect.
Does donor age affect GVHD risk?
Yes. Younger donors are preferred as older donor age correlates with higher GVHD incidence and severity.
The prevention of graft-versus-host disease requires a multifaceted approach incorporating optimal donor selection, personalized immunosuppressive regimens, and careful post-transplant monitoring. Through risk stratification and targeted prophylaxis, we can reduce the burden of GVHD and improve outcomes for allogeneic stem cell transplant recipients.