Cisplatin remains a cornerstone chemotherapeutic agent for treating various solid tumors, including testicular, ovarian, head and neck, and lung cancers. However, its clinical efficacy is compromised by dose-limiting toxicities, notably ototoxicity, which manifests as irreversible, bilateral sensorineural hearing loss. The incidence of cisplatin-induced ototoxicity (CIO) varies from 20% to over 60%, with children and the elderly being particularly susceptible. Effective prevention of CIO is essential for preserving the quality of life of cancer survivors.
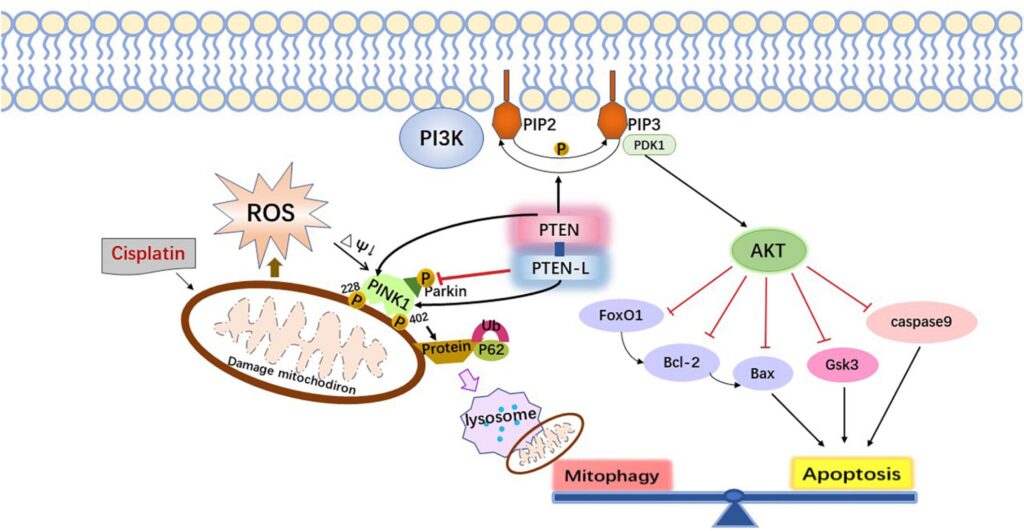
Mechanisms of Cisplatin-Induced Ototoxicity
Cisplatin exerts its cytotoxic effect by forming DNA adducts, triggering apoptosis in rapidly dividing cells. However, it also accumulates in the cochlear hair cells, stria vascularis, and spiral ganglion neurons of the inner ear through passive diffusion and active transport mechanisms, notably via the organic cation transporter 2 (OCT2) and copper transporter 1 (CTR1).
Pathophysiological Features
- Reactive oxygen species (ROS) generation
- Mitochondrial dysfunction
- Inflammatory cytokine release
- Apoptosis of outer hair cells
- Loss of cochlear microvascular supply
Damage predominantly affects the basal turn of the cochlea, leading to high-frequency hearing loss, which may progress to involve lower frequencies.
Risk Factors for Cisplatin-Induced Ototoxicity
Understanding the key predisposing factors facilitates early intervention and risk stratification.
| Risk Factor | Impact |
|---|---|
| Cumulative cisplatin dose | Higher total dose increases ototoxic potential |
| Age (especially <5 or >65 years) | Immature or declining cochlear resilience |
| Pre-existing hearing impairment | Greater susceptibility to further auditory damage |
| Concurrent ototoxic medications | Additive toxicity (e.g., aminoglycosides, loop diuretics) |
| Genetic predisposition (e.g., TPMT, GSTP1 polymorphisms) | Alters drug metabolism and ROS defense mechanisms |
| Renal dysfunction | Impaired drug clearance elevates cisplatin exposure |
Pharmacological Strategies for Otoprotection
1. Sodium Thiosulfate (STS)
STS is the only FDA-approved otoprotective agent for pediatric use in specific cancer contexts.
- Mechanism: Detoxifies cisplatin by forming inactive complexes and scavenging free radicals.
- Timing: Administered 6 hours post-cisplatin infusion to prevent interference with anticancer activity.
- Clinical Evidence: The ACCL0431 trial demonstrated a 48% reduction in ototoxicity in children receiving STS.
2. Amifostine
- Mechanism: Prodrug that is converted intracellularly into a thiol metabolite, protecting normal tissues.
- Limitations: Risk of hypotension, nausea, and uncertain efficacy in preventing CIO.
3. N-Acetylcysteine (NAC)
- Mechanism: Serves as an antioxidant precursor to glutathione.
- Status: Mixed results in trials; not standard of care.
4. D-Methionine
- Mechanism: Free radical scavenger; preserves cochlear glutathione levels.
- Preclinical success, but large-scale human trials are pending.
Cisplatin Dose Modulation and Scheduling
Adjusting cisplatin dosage and infusion duration is crucial to reducing cochlear damage without compromising antitumor efficacy.
- Dose Splitting: Dividing total dose over multiple days reduces peak plasma levels.
- Infusion Time: Prolonged infusion over 6–24 hours leads to slower drug accumulation in cochlear tissues.
- Cumulative Dose Limit: Total cisplatin exposure should ideally not exceed 400 mg/m².
Monitoring and Early Detection
Audiological Monitoring Protocol
A robust audiological surveillance plan is essential to detect early signs of hearing loss.
| Time Point | Recommended Assessment |
|---|---|
| Baseline (pre-treatment) | Pure-tone audiometry, high-frequency audiometry |
| During treatment | Every 1–2 cycles |
| Post-treatment | Every 3 months for first year |
- Otoacoustic emissions (OAE): Useful in children or those unable to complete standard tests
- Extended high-frequency audiometry: Detects early cochlear damage beyond conventional ranges
Non-Pharmacological Interventions
Limiting Concurrent Ototoxins
Avoid or minimize co-administration of known ototoxic drugs such as:
- Aminoglycosides
- Loop diuretics (furosemide)
- Vancomycin
If unavoidable, schedule these agents well apart from cisplatin infusion.
Hydration and Renal Protection
Enhanced renal clearance via pre- and post-hydration protocols (2–3 L/day of isotonic fluids) indirectly reduces cochlear accumulation of cisplatin by facilitating systemic clearance.
Future and Emerging Therapies
Gene Therapy and Molecular Interventions
Targeted gene therapy aims to upregulate anti-apoptotic pathways (e.g., Bcl-2) or ROS scavenging enzymes (e.g., SOD1) within the cochlea. While promising in animal models, these interventions are years away from clinical translation.
Local Intratympanic Drug Delivery
Administering otoprotective agents directly to the middle ear bypasses systemic circulation, preserving chemotherapy efficacy while protecting the cochlea.
- Agents under study: STS, dexamethasone, NAC
- Delivery systems: Gels, nanoparticles, microcatheters
Frequently Asked Questions
Can cisplatin ototoxicity be reversed?
No, hearing loss from cisplatin is generally permanent. Prompt detection and intervention are key to limiting progression.
What is the most effective strategy for preventing ototoxicity?
Sodium thiosulfate, administered 6 hours post-cisplatin, has the strongest evidence, particularly in pediatric populations.
How can children be monitored for ototoxicity?
Otoacoustic emissions and age-appropriate behavioral audiometry are recommended. Regular follow-ups are crucial.
Is there a safe cisplatin dose threshold?
A cumulative dose above 400 mg/m² is associated with a significant rise in ototoxicity risk. Lower thresholds apply in high-risk individuals.
Does hydration help reduce hearing loss?
Yes, although primarily intended for nephroprotection, hydration facilitates cisplatin clearance and may reduce ototoxicity indirectly.
The prevention of cisplatin-induced ototoxicity requires a multifaceted strategy encompassing pharmacological agents, dosing alterations, vigilant monitoring, and avoidance of synergistic toxins. Sodium thiosulfate currently represents the most validated intervention, particularly in the pediatric setting. Personalized approaches based on genetic predisposition, age, and comorbidities are essential to optimize outcomes. As research advances, novel methods such as intratympanic therapy and gene modulation hold promise for more targeted cochlear protection.