Peptococcus osteomyelitis is a rare form of anaerobic bone infection caused by Peptococcus species—gram-positive, non-spore-forming, anaerobic cocci. These bacteria are part of the normal flora in the skin, oral cavity, gastrointestinal tract, and genitourinary tract but may become opportunistic pathogens under conducive conditions such as trauma, surgery, or immunosuppression. Their involvement in osteomyelitis signifies a complex, often polymicrobial infection that demands prompt recognition and treatment.
Pathophysiology and Mechanism of Bone Invasion
The onset of Peptococcus osteomyelitis typically follows direct inoculation, hematogenous dissemination, or contiguous spread from nearby infected tissues. The anaerobic nature of Peptococcus favors low-oxygen environments such as necrotic bone, where they proliferate and provoke chronic inflammation and suppuration.
Common Risk Factors and Predisposing Conditions
Peptococcus osteomyelitis is most prevalent among individuals with pre-existing vulnerabilities that disrupt bone integrity or immune defense.
Predisposing Factors:
- Open fractures or orthopedic implants
- Post-surgical complications
- Diabetic foot ulcers
- Peripheral vascular disease
- Intravenous drug use
- Dental or periodontal infections (especially in craniofacial cases)
- Immunocompromised states (HIV, malignancy, long-term steroids)
Clinical Presentation of Anaerobic Osteomyelitis
Peptococcus-related osteomyelitis often presents as a chronic, indolent infection, with symptoms developing over weeks to months. Due to its nonspecific presentation, diagnosis may be delayed unless anaerobic pathogens are specifically considered.
Typical Clinical Signs:
- Localized bone pain and swelling
- Erythema and warmth over affected area
- Sinus tract formation with foul-smelling purulent discharge
- Fever (inconsistent; may be absent in chronic cases)
- Reduced limb mobility or weight-bearing difficulty
- Systemic signs in advanced infection (fatigue, malaise)
Diagnostic Approach: Identifying Peptococcus as the Etiological Agent
Early and accurate diagnosis is essential to manage Peptococcus osteomyelitis effectively. A multidisciplinary diagnostic approach that combines imaging, microbiology, and histopathology is recommended.
Imaging Studies:
- Plain Radiography
- May show osteolytic lesions, periosteal reaction, or sequestra in late stages
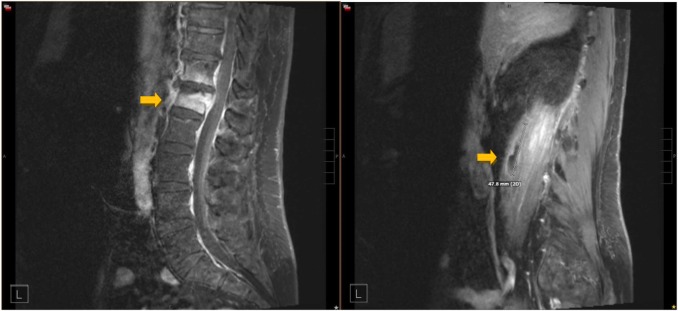
- MRI
- Gold standard for early detection
- Identifies marrow edema, abscesses, and soft tissue involvement
- CT Scan
- Useful in detecting cortical bone destruction and guiding biopsies
- Bone Scintigraphy (Tc-99m or Gallium scans)
- Enhances detection of active infection sites
Laboratory Investigations:
- Elevated ESR and CRP
- White blood cell count: Usually elevated
- Blood cultures: May yield anaerobes, but sensitivity is low for Peptococcus
Microbiological Confirmation:
- Bone biopsy and culture
- Preferably under sterile conditions using anaerobic transport media
- Culture incubation must accommodate anaerobes
- Identification of Peptococcus requires advanced anaerobic culturing, Gram staining (Gram-positive cocci in clusters or chains), and biochemical profiling
Differential Diagnosis of Osteomyelitis with Anaerobic Etiology
Correct identification of the pathogen is vital to avoid mismanagement. The differential diagnosis includes:
| Condition | Distinctive Features |
|---|---|
| Staphylococcal Osteomyelitis | Acute onset, common in children and adults |
| Tubercular Osteomyelitis | Chronic, cold abscess formation, TB contact history |
| Fungal Osteomyelitis | Immunocompromised hosts, slow progressive course |
| Actinomycosis of Bone | Sulfur granules, cervicofacial common |
Treatment Protocol: Managing Peptococcus Osteomyelitis
Successful treatment of Peptococcus osteomyelitis necessitates a combination of prolonged antibiotic therapy and surgical debridement.
Antibiotic Therapy
Initial empirical therapy should provide broad anaerobic coverage. Once culture confirms Peptococcus, antibiotic therapy should be tailored.
Preferred Antibiotics:
- Clindamycin: Excellent anaerobic bone penetration (600–900 mg IV every 8 hours)
- Metronidazole: Effective against obligate anaerobes (500 mg IV/PO every 8 hours)
- Penicillin G: High-dose IV formulation for 4–6 weeks (especially for susceptible strains)
- Beta-lactam/beta-lactamase inhibitors: Piperacillin-tazobactam or ampicillin-sulbactam
Duration of Therapy:
- Typically 6 weeks of intravenous therapy, followed by 4–6 weeks oral antibiotics depending on clinical and radiological response
Surgical Management
Surgery plays a vital role in managing chronic or refractory cases:
- Debridement of necrotic bone and soft tissue
- Removal of hardware or foreign bodies
- Sinus tract excision
- Staged reconstruction for bone defects (e.g., bone grafts, antibiotic cement spacers)
Prognosis and Expected Outcomes
The prognosis of Peptococcus osteomyelitis is favorable with early detection, targeted antimicrobial therapy, and timely surgical intervention.
Positive Prognostic Indicators:
- Early microbiological identification
- Adequate anaerobic antibiotic coverage
- Complete surgical debridement
- Patient compliance with long-term therapy
Complications of Delayed or Inadequate Treatment:
- Chronic osteomyelitis with bone necrosis
- Recurrent infections
- Septicemia
- Amputation in severe or unresponsive cases
- Osteolysis with pathologic fractures
Preventive Measures and Long-Term Monitoring
Prevention of Peptococcus osteomyelitis centers on addressing modifiable risk factors and ensuring sterile technique during surgeries or invasive procedures.
Preventive Strategies:
- Meticulous surgical asepsis
- Prompt treatment of soft tissue infections
- Glycemic control in diabetics
- Dental hygiene maintenance
- Antibiotic prophylaxis for high-risk procedures
Follow-Up and Monitoring:
- Serial imaging to confirm resolution
- ESR and CRP for monitoring inflammation
- Regular wound inspection in postoperative or chronic care settings
- Functional rehabilitation for affected limbs
Peptococcus osteomyelitis, though uncommon, represents a serious anaerobic bone infection that requires high clinical vigilance. Its insidious onset and polymicrobial nature demand a methodical diagnostic and therapeutic strategy. Through a combination of advanced imaging, anaerobic cultures, targeted antibiotics, and surgical debridement, successful outcomes can be achieved. Comprehensive patient management and long-term monitoring remain key to preventing recurrence and minimizing morbidity.