Cervical cancer, driven predominantly by persistent human papillomavirus (HPV) infection, remains a leading cause of cancer-related mortality in women worldwide. The identification of Programmed Death-Ligand 1 (PD-L1) expression in cervical tumors has introduced a pivotal biomarker for patient selection in immune checkpoint therapy. PD-L1 positive cervical cancer represents a biologically distinct entity with unique immune microenvironment features, altering the therapeutic landscape.
Molecular Profile and Immunologic Landscape of PD-L1 Positive Cervical Tumors
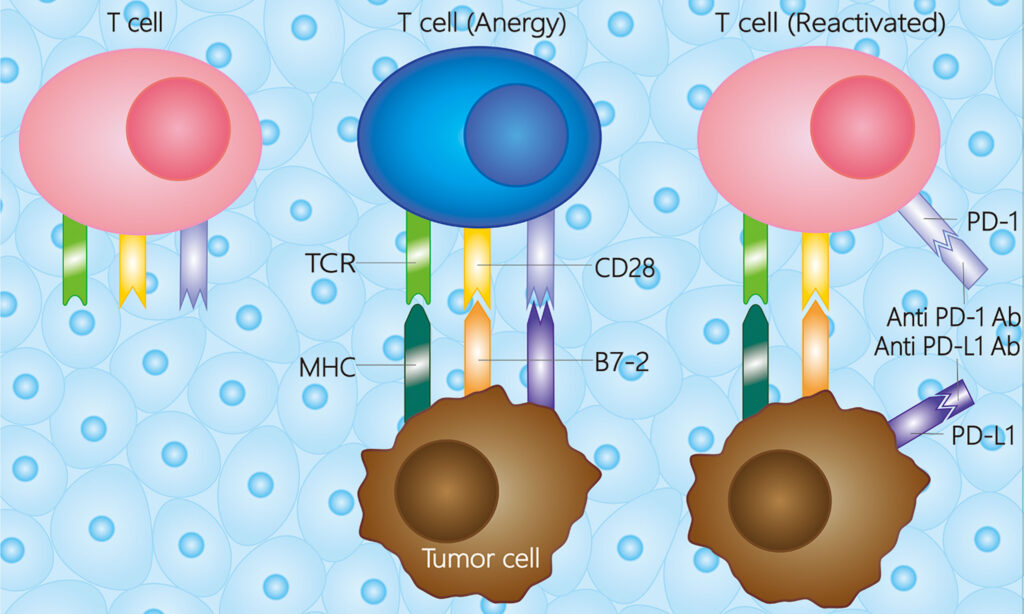
PD-L1 Expression and Tumor Immune Evasion
PD-L1 is a cell surface protein that binds to PD-1 on T lymphocytes, inhibiting cytotoxic activity and promoting immune tolerance. In cervical cancer, PD-L1 is frequently upregulated due to HPV-mediated oncogenic signaling, inflammatory stimuli, and interferon-γ exposure.
Key features of PD-L1 positive cervical cancer:
- High PD-L1 expression on both tumor cells (TCs) and tumor-associated immune cells (ICs)
- Enrichment of tumor-infiltrating lymphocytes (TILs)
- Association with immune-active or inflamed tumor microenvironment (TME)
Prevalence and Demographics
PD-L1 expression is detected in approximately 55%–88% of cervical cancer cases, with variation based on:
- Histology (squamous cell carcinomas more likely than adenocarcinomas)
- HPV subtype (HPV-16 and HPV-18 linked to increased PD-L1)
- Tumor grade and stage (higher PD-L1 in advanced or recurrent disease)
Diagnostic Strategies for PD-L1 Testing in Cervical Cancer
Immunohistochemistry and Combined Positive Score (CPS)
PD-L1 is evaluated via immunohistochemistry (IHC), most commonly using the 22C3 pharmDx assay, which yields a Combined Positive Score (CPS) — the number of PD-L1–positive cells (tumor and immune) divided by total viable tumor cells × 100.
| CPS Score | Interpretation |
|---|---|
| CPS < 1 | PD-L1 Negative |
| CPS ≥ 1 | PD-L1 Positive |
| CPS ≥ 10 | Higher likelihood of benefit |
The KEYNOTE-158 trial established CPS ≥1 as the threshold for pembrolizumab monotherapy eligibility in recurrent or metastatic cervical cancer.
FDA-Approved Immunotherapy in PD-L1 Positive Cervical Cancer
Pembrolizumab (Keytruda)
Pembrolizumab, a PD-1 inhibitor, is the first FDA-approved immune checkpoint inhibitor for PD-L1 positive cervical cancer, supported by:
- KEYNOTE-158 Trial: Demonstrated 14.3% overall response rate (ORR) in PD-L1 positive patients
- KEYNOTE-826 Trial: Pembrolizumab with chemotherapy ± bevacizumab improved progression-free survival (PFS) and overall survival (OS)
Indications:
- Recurrent or metastatic cervical cancer with CPS ≥1
- Used in combination with paclitaxel + cisplatin/carboplatin ± bevacizumab
Cemiplimab (Libtayo)
Cemiplimab, another PD-1 inhibitor, has demonstrated survival benefit as second-line monotherapy in recurrent cervical cancer regardless of PD-L1 status but shows enhanced efficacy in PD-L1 positive tumors.
Treatment Algorithms for PD-L1 Positive Cervical Cancer
First-Line Treatment Approach
Patients with PD-L1 CPS ≥1 and recurrent/metastatic disease:
- Pembrolizumab + Chemotherapy ± Bevacizumab
- Backbone chemotherapy includes paclitaxel + platinum agent
- Bevacizumab added in eligible patients
Second-Line and Beyond
For progression after chemotherapy:
- Pembrolizumab monotherapy (if not used previously)
- Cemiplimab as monotherapy
- Clinical trials evaluating novel checkpoint inhibitor combinations
Predictive Biomarkers and Resistance Mechanisms
Beyond PD-L1: Additional Predictive Markers
Although PD-L1 remains the cornerstone, other factors modulate immunotherapy response:
- Tumor Mutational Burden (TMB): High TMB correlates with improved immunotherapy response
- Microsatellite Instability (MSI): Rare in cervical cancer but indicative of checkpoint blockade sensitivity
- Gene expression profiling: Immune-related mRNA signatures linked to favorable outcomes
Resistance Pathways
Immune evasion in PD-L1 positive cervical tumors may arise due to:
- Loss of MHC Class I expression
- Exclusion of T-cells from TME
- Upregulation of other inhibitory checkpoints (e.g., TIGIT, LAG-3)
- Immunosuppressive stromal and myeloid cells
Overcoming resistance requires combination therapies, novel immune modulators, and TME reprogramming agents.
Emerging Immunotherapy Combinations and Trials
Innovative strategies under investigation:
- Checkpoint inhibitor combinations: PD-1 + CTLA-4 blockade (e.g., nivolumab + ipilimumab)
- Therapeutic cancer vaccines: Targeting HPV E6/E7 oncoproteins
- Adoptive T-cell therapy (TILs): Promising results in HPV-driven tumors
- Oncolytic viruses and STING agonists: Promote innate immune activation
These trials aim to expand benefit to PD-L1 negative tumors and boost durable responses.
Prognostic Implications and Long-Term Outcomes
PD-L1 positivity serves both as a predictive biomarker for immunotherapy and an independent prognostic indicator in certain cervical cancer cohorts. Long-term benefits observed with immune checkpoint blockade include:
- Sustained complete responses
- Improved OS in PD-L1 positive populations
- Better quality of life due to manageable toxicity profiles
Ongoing follow-up from clinical trials will further elucidate survival dynamics in treated cohorts.
PD-L1 positive cervical cancer defines a biologically active and therapeutically targetable subtype. The advent of PD-1 blockade, particularly with pembrolizumab, has reshaped management strategies for advanced disease. Diagnostic precision through PD-L1 testing and strategic application of immunotherapy significantly improves patient outcomes. Continuous evolution of immunomodulatory combinations and personalized biomarkers promises a more effective and durable treatment paradigm for cervical cancer.