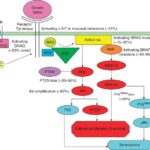
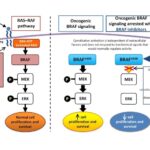
Malignant melanoma with BRAF V600E mutation is a highly aggressive skin cancer caused by a genetic alteration in the BRAF gene, affecting the MAPK/ERK signaling pathway. This mutation leads to uncontrolled cell growth and is found in approximately 80–90% of BRAF-mutated melanomas. Advances in targeted therapy and immunotherapy have significantly improved survival rates, emphasizing the importance of early detection and precise diagnosis.
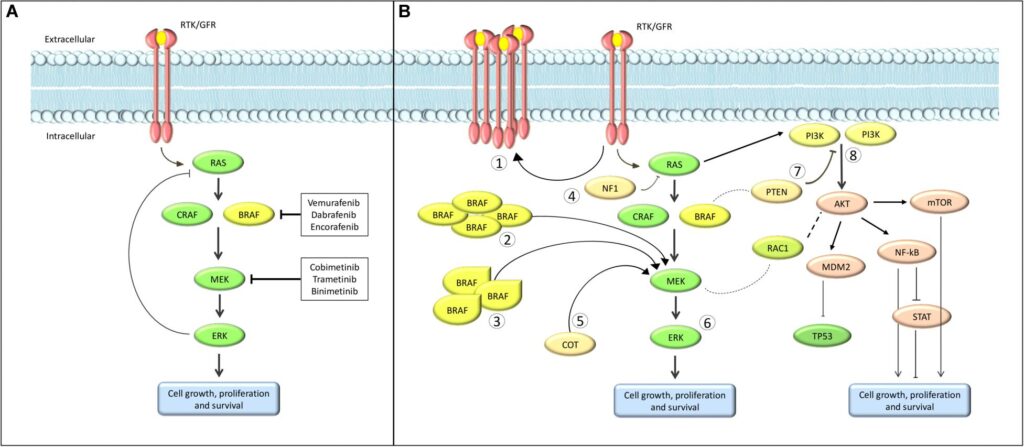
Understanding BRAF V600E Mutation in Melanoma
The BRAF gene encodes a serine/threonine-protein kinase that regulates cell division. The V600E mutation substitutes valine (V) with glutamic acid (E) at codon 600, leading to constant activation of the MAPK/ERK pathway, which drives melanoma progression.
Symptoms of Malignant Melanoma with BRAF V600E Mutation
Melanomas with this mutation exhibit characteristic signs, including:
- Rapid growth of skin lesions
- Irregular borders and asymmetry
- Multiple colors within a single mole
- Diameter larger than 6mm
- Itching, bleeding, or ulceration
- Metastatic spread to lymph nodes, lungs, brain, or liver
Diagnosis of BRAF V600E-Mutant Melanoma
Clinical Examination and Biopsy
- Dermoscopy: Identifies irregular mole structures.
- Excisional Biopsy: Preferred for primary melanoma diagnosis.
- Histopathological Analysis: Confirms malignancy.
Genetic Testing for BRAF V600E Mutation
- Polymerase Chain Reaction (PCR): Detects the mutation.
- Next-Generation Sequencing (NGS): Determines BRAF subtype.
- Immunohistochemistry (IHC): Identifies BRAF protein expression.
Imaging for Staging
- CT, MRI, and PET scans assess metastases.
- Sentinel Lymph Node Biopsy (SLNB) evaluates regional lymph node involvement.
Treatment Strategies for BRAF V600E-Mutant Melanoma
Targeted Therapy: BRAF and MEK Inhibitors
BRAF inhibitors specifically block the mutated BRAF protein, while MEK inhibitors prevent downstream signaling, reducing tumor resistance.
FDA-Approved BRAF/MEK Inhibitor Combinations
- Dabrafenib (BRAF) + Trametinib (MEK)
- Vemurafenib (BRAF) + Cobimetinib (MEK)
- Encorafenib (BRAF) + Binimetinib (MEK)
Immunotherapy for BRAF V600E-Mutant Melanoma
Checkpoint inhibitors enhance the immune system’s ability to fight cancer.
- PD-1 Inhibitors: Pembrolizumab, Nivolumab
- CTLA-4 Inhibitor: Ipilimumab
- Combination Therapy: PD-1 + CTLA-4 inhibitors for advanced cases
Surgical and Radiation Interventions
- Wide Local Excision (WLE): Removes primary tumors.
- Lymphadenectomy: Removes affected lymph nodes.
- Radiotherapy: Used for brain metastases and in palliative care.
Prognosis and Survival Rates
- Localized (Stage I-II): 5-year survival rate of 80–90%.
- Regional (Stage III): Survival rates of 50–70%.
- Metastatic (Stage IV): Median survival has improved significantly with modern treatments.
Preventive Measures and Future Research
- Sun protection with broad-spectrum sunscreen and protective clothing.
- Regular skin exams for early detection.
- Ongoing clinical trials for mRNA vaccines and next-generation immunotherapies.
Malignant melanoma with BRAF V600E mutation is an aggressive but treatable cancer. Targeted therapies and immunotherapies have revolutionized treatment, improving survival rates. Early detection and genetic testing remain key to effective management and better patient outcomes.