Gastric and gastroesophageal junction (GEJ) adenocarcinomas are aggressive malignancies with limited treatment options. Recent advancements have identified CLDN18.2 as a promising biomarker in patients with HER2-negative tumors, offering new therapeutic possibilities.
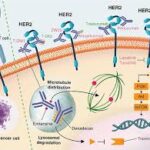
Understanding HER2-Negative Gastric Cancer
HER2-negative gastric cancer refers to tumors that lack overexpression of the human epidermal growth factor receptor 2 (HER2) protein. These cancers are typically more difficult to treat with targeted therapies designed for HER2-positive malignancies.
Characteristics of HER2-Negative Tumors
- Absence of HER2 overexpression confirmed through immunohistochemistry (IHC) and in situ hybridization (ISH).
- Typically diagnosed at advanced stages.
- Often associated with poor prognosis and limited targeted treatment options.
CLDN18.2: A Critical Biomarker
Claudin 18.2 (CLDN18.2) is a tight junction protein selectively expressed in gastric mucosal cells. It becomes exposed during malignant transformation, making it an accessible target for immunotherapies.
Role of CLDN18.2 in Gastric Cancer
- Found in approximately 30-40% of gastric and GEJ adenocarcinomas.
- Strong association with diffuse-type gastric cancer.
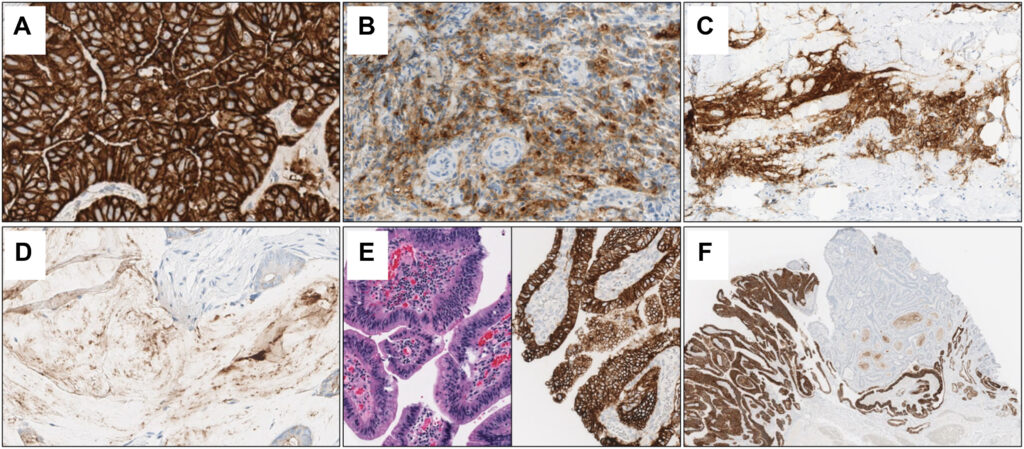
- Identified through IHC testing with specific monoclonal antibodies.
Diagnostic Techniques for CLDN18.2
Identifying CLDN18.2 expression is crucial for selecting targeted therapies. Diagnostic procedures include:
- Immunohistochemistry (IHC): Standard method to detect CLDN18.2 expression.
- Molecular Profiling: Used for deeper analysis when IHC results are inconclusive.
Treatment Strategies for HER2-Negative and CLDN18.2-Positive Adenocarcinoma
Monoclonal Antibody Therapy
Emerging treatments target CLDN18.2 to improve outcomes in HER2-negative patients.
- Zolbetuximab (IMAB362): A monoclonal antibody designed to bind CLDN18.2, triggering immune response and direct tumor cell destruction.
Chemotherapy in Combination
Clinical trials demonstrate improved progression-free survival (PFS) and overall survival (OS) when combining CLDN18.2-targeting agents with chemotherapy.
Immunotherapy Advancements
- Checkpoint Inhibitors: Exploring synergy between CLDN18.2-targeted therapies and PD-1/PD-L1 inhibitors to enhance immune response.
Clinical Trials and Emerging Data
Ongoing research continues to evaluate the efficacy of CLDN18.2-targeting treatments. Studies like the SPOTLIGHT trial and GLOW trial have shown encouraging results in metastatic gastric/GEJ cancer patients.
Key Findings
- SPOTLIGHT Trial: Demonstrated significant improvement in median PFS and OS in CLDN18.2-positive patients treated with Zolbetuximab.
- GLOW Trial: Reinforced the positive impact of combining targeted therapy with standard chemotherapy regimens.
Prognosis and Survival Rates
Patients with HER2-negative and CLDN18.2-positive tumors typically face poorer outcomes without targeted therapies. However, with advances in CLDN18.2-directed treatments, survival rates are improving significantly.
Prognostic Factors
- Stage at diagnosis.
- Tumor burden and metastasis extent.
- Response to emerging therapies like Zolbetuximab.
Future Directions and Research
Research continues to explore additional immunotherapy combinations, novel CLDN18.2-targeted agents, and biomarker-driven treatment strategies to improve outcomes for this patient subgroup.
FAQs
What is CLDN18.2?
CLDN18.2 is a protein expressed in gastric cells that becomes an actionable target in certain gastric cancers.
How is CLDN18.2 detected?
CLDN18.2 expression is typically identified using immunohistochemistry (IHC) testing.
Is Zolbetuximab approved for clinical use?
Zolbetuximab has shown promising results in clinical trials, but regulatory approvals may vary by region.
Are there alternative therapies for CLDN18.2-positive patients?
Combination strategies involving chemotherapy and immunotherapy are being actively explored in clinical trials.
What is the prognosis for patients with HER2-negative and CLDN18.2-positive cancer?
Prognosis varies based on treatment response, but targeted therapies like Zolbetuximab show promising improvements in survival outcomes.
The identification of CLDN18.2 as a therapeutic target has revolutionized the treatment landscape for HER2-negative gastric and gastroesophageal junction adenocarcinomas. With targeted therapies such as Zolbetuximab and ongoing research, improved outcomes are increasingly achievable for this challenging cancer subtype.