Tuberculosis (TB), caused by Mycobacterium tuberculosis, remains a major global health challenge. Early detection is essential for effective control and treatment. One of the primary diagnostic tools is the delayed hypersensitivity skin test, also known as the Mantoux test, which utilizes tuberculin purified protein derivative (PPD) to detect prior exposure to M. tuberculosis. This article provides an in-depth examination of the test, including its methodology, interpretation, influencing factors, and clinical significance.
Understanding Delayed Hypersensitivity and the PPD Test
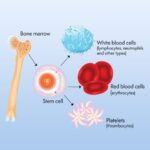
The delayed hypersensitivity reaction, or Type IV hypersensitivity, is a T-cell mediated immune response. When an individual sensitized to a specific antigen is re-exposed, T-lymphocytes release cytokines that trigger localized inflammation.
In the PPD skin test, this reaction manifests as induration (hardening of the skin) at the test site. A positive response indicates prior exposure to M. tuberculosis or related mycobacteria but does not differentiate between latent TB infection (LTBI) and active disease.
Mechanism of Delayed Hypersensitivity Reaction
PPD Skin Test Procedure
Test Administration
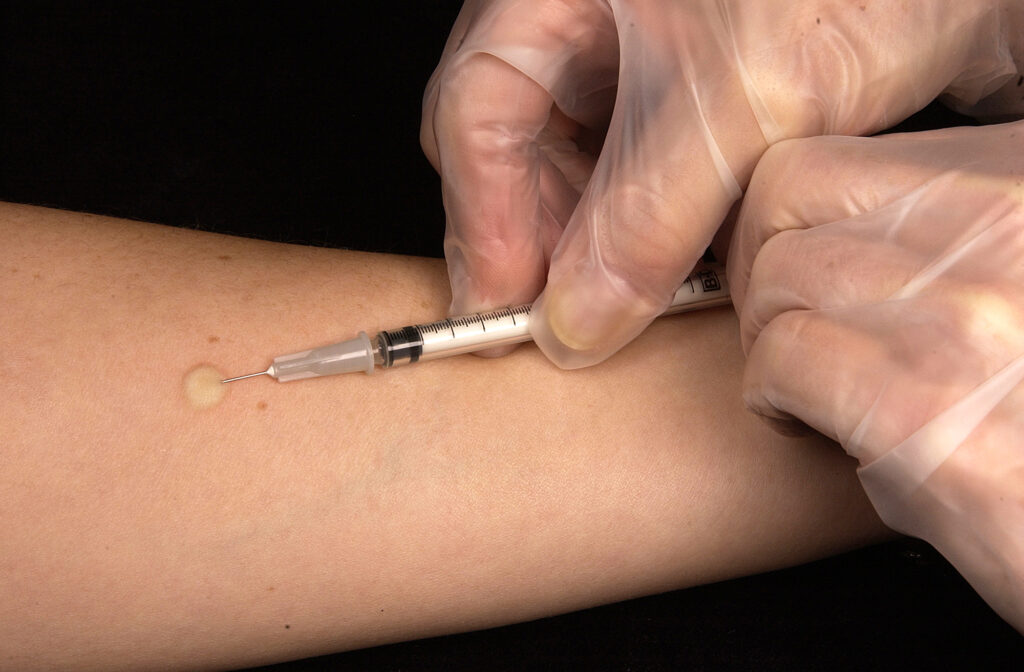
The Mantoux test is performed using an intradermal injection of 0.1 mL of PPD solution (5 tuberculin units). The injection is given on the inner forearm, creating a small wheal (6–10 mm in diameter). Proper technique is critical to ensure accurate results.
Test Interpretation
Results are read 48 to 72 hours after injection, measuring the induration (raised area, not redness) in millimeters. Interpretation depends on risk factors:
| Induration Size | Interpretation |
|---|---|
| ≥5 mm | Positive in high-risk groups: HIV-positive individuals, recent TB contacts, immunosuppressed patients, organ transplant recipients. |
| ≥10 mm | Positive in moderate-risk individuals: healthcare workers, immigrants from TB-endemic regions, IV drug users, laboratory personnel. |
| ≥15 mm | Positive in individuals with no known TB risk factors. |
Factors Affecting Test Accuracy
False Positive Results
A positive test does not necessarily indicate active TB. Potential causes include:
- BCG vaccination (Bacillus Calmette-Guérin)
- Exposure to non-tuberculous mycobacteria
False Negative Results
A negative test does not always rule out TB infection. Possible reasons include:
Clinical Significance of the PPD Test
The PPD test is a cornerstone of TB screening and is essential for:
- Identifying latent TB infections (LTBI)
- Screening high-risk individuals before immunosuppressive therapy
- Preventing TB transmission through early intervention
However, a positive result requires further evaluation, including chest X-ray, sputum culture, and molecular tests, to confirm active TB.