Acute Myeloid Leukemia with Isocitrate Dehydrogenase-1 (AML) is a heterogeneous hematologic malignancy characterized by the clonal proliferation of myeloid progenitor cells. Among the various genetic alterations associated with AML, mutations in the isocitrate dehydrogenase-1 (IDH1) gene have garnered significant attention due to their impact on disease pathogenesis and prognosis. These mutations are present in approximately 6% to 10% of AML cases and are associated with distinct metabolic and epigenetic changes that influence disease progression and response to therapy.
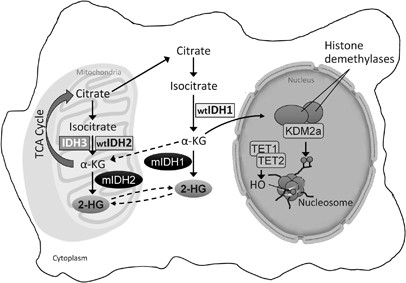
Pathophysiology of IDH1 Mutations in AML
IDH1 is an enzyme involved in the citric acid cycle, converting isocitrate to alpha-ketoglutarate. Mutations in the IDH1 gene result in a neomorphic enzyme activity that produces D-2-hydroxyglutarate (D-2HG), an oncometabolite that accumulates within the cell. Elevated levels of D-2HG inhibit alpha-ketoglutarate-dependent enzymes, leading to DNA and histone hypermethylation. This epigenetic alteration disrupts normal gene expression, promoting leukemogenesis by impairing cellular differentiation and enhancing proliferation.
Clinical Implications
Prognostic Significance
The presence of IDH1 mutations in AML patients is associated with a poor prognosis, particularly in cases with normal cytogenetics. These mutations are linked to increased DNA methylation, which can lead to aberrant gene expression patterns that favor leukemic cell survival and proliferation.
Diagnostic Considerations
Accurate detection of IDH1 mutations is crucial for prognostication and therapeutic decision-making. Diagnostic approaches include:
- Molecular Testing: Polymerase chain reaction (PCR) and next-generation sequencing (NGS) are employed to identify specific IDH1 mutations, such as the R132H variant.
- Immunohistochemistry: Antibodies targeting mutant IDH1 proteins can be used to detect the presence of mutations in tissue samples.
- Metabolite Analysis: Elevated levels of D-2HG in blood or bone marrow aspirates can serve as a biomarker for IDH1 mutations.
Treatment Strategies
Targeted Therapy
The development of IDH1 inhibitors has revolutionized the treatment landscape for IDH1-mutated AML. These agents specifically inhibit the mutant IDH1 enzyme, reducing D-2HG levels and reversing epigenetic modifications.
- Ivosidenib (Tibsovo): Approved by the U.S. Food and Drug Administration (FDA) in July 2018, ivosidenib is an oral IDH1 inhibitor indicated for relapsed or refractory AML with a susceptible IDH1 mutation. Clinical trials have demonstrated that ivosidenib monotherapy can induce remission in a subset of patients. en.wikipedia.org
- Olutasidenib (Rezlidhia): In December 2022, the FDA approved olutasidenib for the treatment of relapsed or refractory AML with a susceptible IDH1 mutation. This agent offers an alternative for patients who may not respond to ivosidenib. en.wikipedia.org
Combination Therapy
Combining IDH1 inhibitors with other therapeutic modalities has shown promise in enhancing treatment efficacy:
- Ivosidenib and Azacitidine: A study published in the New England Journal of Medicine in 2022 reported that the combination of ivosidenib and the hypomethylating agent azacitidine resulted in higher remission rates compared to azacitidine alone in patients with IDH1-mutated AML. nejm.org
- Ivosidenib and Chemotherapy: Research indicates that integrating ivosidenib with standard chemotherapy regimens may improve outcomes for patients with IDH1-mutated AML. cancer.gov
Chemotherapy
Traditional chemotherapy remains a cornerstone in the treatment of AML. For patients with IDH1 mutations, chemotherapy can be combined with IDH1 inhibitors to enhance efficacy. The choice of chemotherapy regimen depends on patient-specific factors, including age, performance status, and comorbidities.
Prognosis and Future Directions
The advent of targeted therapies has improved the prognosis for patients with IDH1-mutated AML. Ongoing clinical trials are exploring the efficacy of combining IDH1 inhibitors with other agents, such as immune checkpoint inhibitors and novel chemotherapeutic agents, to further enhance treatment outcomes. Personalized treatment approaches, guided by genetic profiling, are expected to become standard practice, offering tailored therapies that address the unique molecular characteristics of each patient’s leukemia.
IDH1 mutations play a pivotal role in the pathogenesis of acute myeloid leukemia, influencing disease progression and response to therapy. Advancements in molecular diagnostics and targeted therapies have significantly improved the management of IDH1-mutated AML. Continued research is essential to fully understand the implications of IDH1 mutations and to develop more effective, personalized treatment strategies for patients affected by this challenging malignancy.