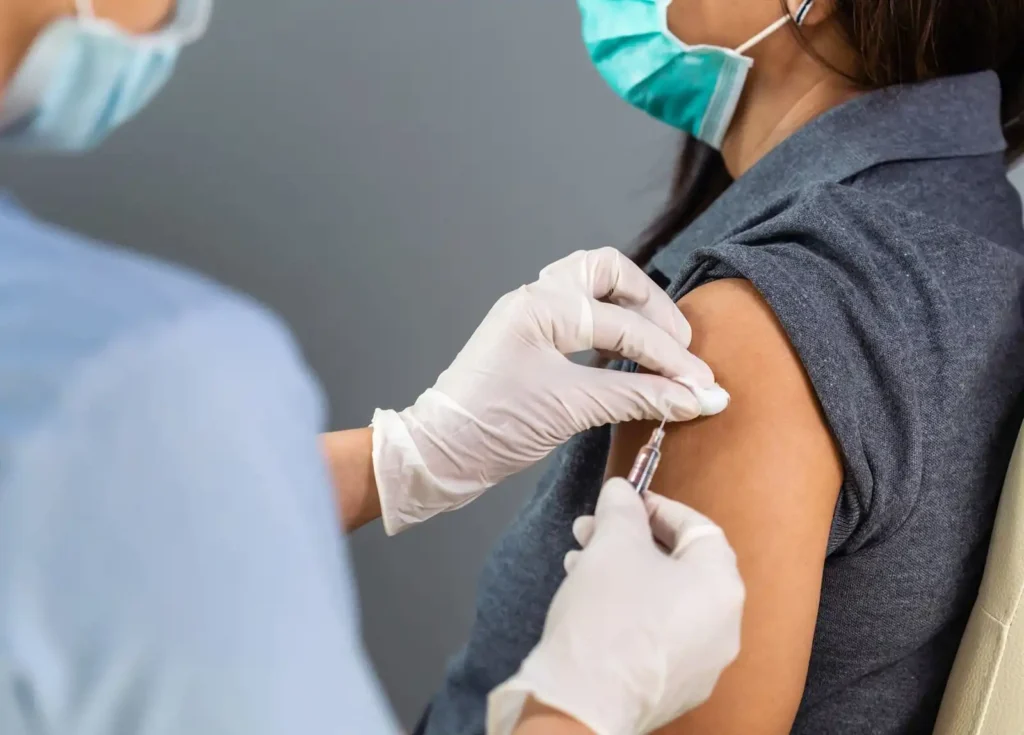
Chikungunya virus, a mosquito-borne alphavirus, has emerged as a significant public health concern globally. With no specific antiviral treatment available, vaccination remains the most promising solution for prevention. In this detailed article, we explore the advancements in chikungunya virus vaccination, its importance, and the current status of vaccine development.
Understanding Chikungunya Virus
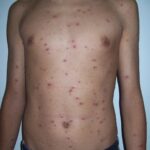
Chikungunya virus (CHIKV) is transmitted primarily by Aedes aegypti and Aedes albopictus mosquitoes. It causes chikungunya fever, characterized by high fever, joint pain, headache, rash, and fatigue. Severe cases can lead to chronic arthritis, which significantly affects the quality of life.
Global Impact of Chikungunya
The virus has caused outbreaks across Africa, Asia, Europe, and the Americas. Its rapid spread and debilitating symptoms underscore the urgent need for effective vaccines.
Why Vaccination is Critical
Limitations of Current Prevention Methods
Efforts to control chikungunya rely on vector management and personal protection. However, these measures have limited efficacy in endemic regions with dense mosquito populations.
Herd Immunity and Public Health Benefits
Vaccination can not only protect individuals but also contribute to herd immunity, significantly reducing disease transmission.
Current Landscape of Chikungunya Vaccination
Types of Vaccines Under Development
- Live-Attenuated Vaccines
- Use a weakened form of the virus.
- Show strong immune response but may pose safety concerns.
- Inactivated Vaccines
- Utilize killed viruses.
- Safer but often require multiple doses.
- Virus-Like Particle (VLP) Vaccines
- Mimic the virus structure without containing genetic material.
- Offer high immunogenicity and safety.
- mRNA-Based Vaccines
- Leverage cutting-edge technology.
- Rapid production with a strong immune response.
- DNA Vaccines
- Use DNA to encode viral proteins.
- Stable and cost-effective for large-scale production.
Key Vaccine Candidates
Several promising vaccine candidates are undergoing clinical trials, including:
- VLA1553 (Valneva SE): A live-attenuated vaccine in advanced clinical trials.
- CHIKV-VLP: A virus-like particle vaccine demonstrating strong immunogenicity.
- mRNA-1388: Moderna’s mRNA-based candidate showing encouraging results.
Challenges in Vaccine Development
Genetic Diversity of CHIKV
The virus exhibits genetic variations, complicating vaccine design to ensure broad efficacy.
Safety and Efficacy Testing
Ensuring the safety and long-term efficacy of vaccines remains a critical step before regulatory approval.
Cost and Accessibility
Developing vaccines that are affordable and accessible, especially in low-income regions, is vital for global health impact.
Future Prospects
Integration with Global Vaccination Programs
Chikungunya vaccines have the potential to be integrated into existing immunization schedules, enhancing reach and effectiveness.
Focus on Universal Vaccines
Efforts are underway to develop vaccines effective against all chikungunya strains, addressing the challenge of genetic diversity.
Prevention Beyond Vaccination
While vaccines offer a long-term solution, complementary measures are essential:
- Mosquito Control: Using insecticides, larvicides, and environmental management.
- Personal Protection: Wearing long sleeves, using mosquito repellents, and sleeping under bed nets.
- Community Awareness: Educating the public about prevention and symptoms.