Zidovudine (AZT), a nucleoside reverse transcriptase inhibitor (NRTI), was the first antiretroviral drug approved for the treatment of HIV. While effective in suppressing viral replication, zidovudine is associated with a range of adverse effects, among which anemia is one of the most clinically significant. Zidovudine-induced anemia can limit the drug’s therapeutic potential and compromise treatment adherence and quality of life in HIV-infected individuals.
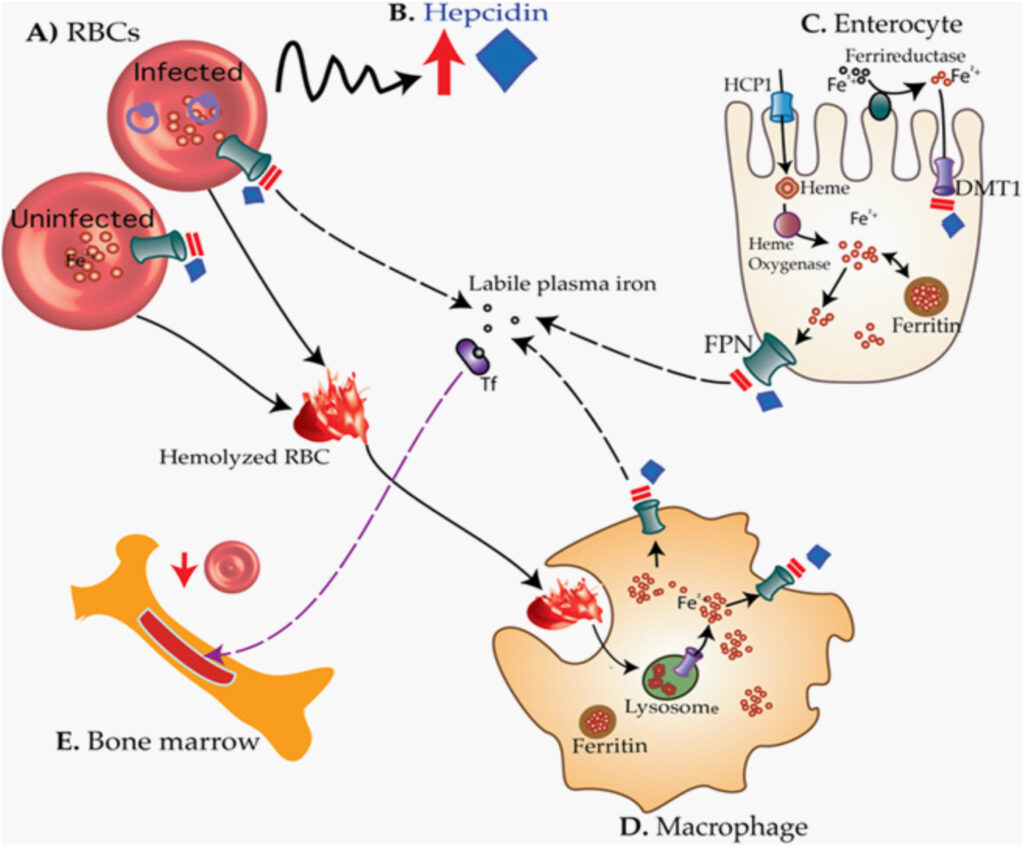
Mechanism of Zidovudine-Induced Anemia
Mitochondrial Toxicity and Erythropoiesis Suppression
Zidovudine interferes with mitochondrial DNA polymerase-γ, leading to impaired mitochondrial replication. The resulting mitochondrial dysfunction affects rapidly dividing cells, especially erythroid precursors in the bone marrow.
Bone Marrow Suppression
Zidovudine’s primary hematologic toxicity manifests as bone marrow suppression, inhibiting erythropoiesis and causing macrocytic anemia.
Dose-Dependent Toxicity
The development of anemia is often dose-related and may be exacerbated in patients receiving higher cumulative doses or those with pre-existing hematological compromise.
Clinical Features of Zidovudine-Induced Anemia
Symptoms
Patients may exhibit classical signs of anemia:
- Fatigue
- Dyspnea on exertion
- Pallor
- Tachycardia
- Dizziness
These symptoms are often insidious and may overlap with the clinical picture of HIV itself, necessitating careful evaluation.
Laboratory Findings
- Hemoglobin (Hb): Decreased, typically <10 g/dL in moderate to severe cases
- Mean Corpuscular Volume (MCV): Elevated (macrocytosis)
- Reticulocyte count: Decreased or inappropriately normal
- Bone marrow examination: Normoblastic or megaloblastic changes with erythroid hypoplasia
Risk Factors for Developing Zidovudine-Induced Anemia
Understanding patient-specific risk factors helps tailor antiretroviral therapy and monitor adverse effects proactively.
Key Risk Factors:
- Advanced HIV/AIDS (low CD4 count)
- Baseline anemia
- Nutritional deficiencies (e.g., B12, folate)
- Renal insufficiency
- Concomitant use of other myelosuppressive drugs
- Prolonged duration of zidovudine therapy
Diagnostic Approach to Zidovudine-Induced Anemia
Anemia in HIV patients must be thoroughly investigated to identify reversible or drug-related causes. Zidovudine-induced anemia is diagnosed by:
- Detailed history including ART regimen and timeline
- Complete blood count (CBC) with red cell indices
- Reticulocyte count and peripheral smear
- Vitamin B12 and folate levels
- Bone marrow biopsy in refractory or unclear cases
The temporal correlation between initiation of zidovudine and the onset of anemia supports the diagnosis.
Management Strategies for Zidovudine-Induced Anemia
Dose Reduction or Discontinuation
The first-line intervention involves reducing the zidovudine dose or substituting it with a less myelosuppressive NRTI such as tenofovir or abacavir.
Supportive Measures
- Blood transfusions for severe symptomatic anemia
- Erythropoietin-stimulating agents (ESAs) in select cases
- Nutritional support for coexistent deficiencies
- Monitoring iron status, though iron overload should be avoided
ART Regimen Optimization
Transitioning to newer antiretroviral combinations with better hematologic tolerability significantly reduces the burden of zidovudine-associated anemia. Personalized therapy based on viral load, resistance patterns, and comorbidities is essential.
Prognosis and Long-Term Considerations
With early recognition and appropriate management, zidovudine-induced anemia is usually reversible. However, delayed intervention can result in:
- Reduced ART adherence due to fatigue
- Impaired immune recovery
- Increased hospitalization
- Deterioration in quality of life
Proactive hematologic monitoring is recommended, especially within the first 8–12 weeks of initiating zidovudine therapy.
Prevention and Clinical Recommendations
Baseline Assessment
Before initiating zidovudine, assess:
- Complete blood count
- Renal function
- Nutritional status
Monitoring Guidelines
| Parameter | Frequency |
|---|---|
| Hemoglobin levels | Every 2–4 weeks initially |
| MCV and reticulocyte count | Monthly |
| ART toxicity review | Each clinic visit |
Early detection enables dose modification before progression to symptomatic anemia.
Comparative Toxicity Profile
| Drug | Anemia Risk | Mechanism |
|---|---|---|
| Zidovudine | High | Bone marrow suppression |
| Tenofovir | Low | Nephrotoxicity (not anemia) |
| Stavudine | Moderate | Mitochondrial toxicity |
| Lamivudine | Minimal | Generally well tolerated |
Zidovudine remains one of the few antiretroviral agents with a significant risk of anemia, underscoring the importance of its judicious use.
Zidovudine-induced anemia is a well-documented complication that demands vigilant clinical attention. While the drug has played a crucial historical role in HIV therapy, its hematologic toxicity can significantly affect treatment success. Early detection, appropriate management, and a shift toward better-tolerated regimens are vital in minimizing morbidity and ensuring optimal patient outcomes.