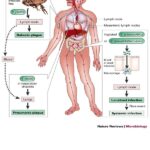
Zaire ebolavirus, a member of the Filoviridae family, is one of the most virulent pathogens responsible for Ebola Virus Disease (EVD), a severe and often fatal hemorrhagic fever. This virus gained notoriety due to multiple deadly outbreaks primarily in Central and West Africa. The Zaire strain (EBOV) is known for its high case fatality rates, often exceeding 50%, posing significant public health challenges worldwide.
Virology and Genetic Characteristics of Zaire Ebolavirus
Zaire ebolavirus is an enveloped, negative-sense single-stranded RNA virus with a filamentous morphology. Its genome encodes seven structural proteins critical for replication, immune evasion, and virulence:
- Nucleoprotein (NP)
- VP35 and VP40 (polymerase co-factors)
- Glycoprotein (GP), responsible for host cell attachment and entry
- VP30 and VP24, involved in transcription and immune suppression
- L protein (RNA-dependent RNA polymerase)
The viral glycoprotein mediates fusion with host cells, primarily targeting monocytes, macrophages, dendritic cells, and endothelial cells, which contributes to systemic dissemination.
Epidemiology and Natural Reservoirs
Zaire ebolavirus is endemic in specific regions of Central Africa, including the Democratic Republic of Congo, Gabon, and the Republic of Congo. It is a zoonotic virus with fruit bats (family Pteropodidae) identified as the primary natural reservoirs.
Transmission Cycle:
- Primary spillover: Human infection occurs through direct contact with infected wildlife, especially bats or non-human primates.
- Human-to-human transmission: Occurs via direct contact with blood, secretions, organs, or other bodily fluids of infected individuals.
- Nosocomial transmission: Improper infection control practices in healthcare settings facilitate spread.
- Fomite transmission: Virus remains viable on surfaces for hours to days, posing indirect transmission risks.
Pathogenesis and Mechanisms of Disease Progression
Zaire ebolavirus enters host cells through macropinocytosis mediated by viral glycoprotein binding. Once inside, it replicates in the cytoplasm and induces a cascade of immune dysregulation.
Key Pathogenic Mechanisms:
- Dysregulated immune response: Infected macrophages and dendritic cells release pro-inflammatory cytokines (cytokine storm), causing systemic inflammation.
- Endothelial damage: Viral replication disrupts vascular integrity, leading to increased permeability and hemorrhage.
- Coagulopathy: Activation of coagulation pathways results in disseminated intravascular coagulation (DIC).
- Multi-organ failure: Viral replication in liver, kidneys, and adrenal glands leads to organ dysfunction.
Clinical Presentation and Symptomatology of Zaire Ebolavirus Infection
The incubation period ranges from 2 to 21 days. The illness progresses in phases:
Early Symptoms (Days 1–7):
- Sudden onset of high fever
- Severe headache and myalgia
- Fatigue and malaise
- Pharyngitis and gastrointestinal symptoms (nausea, vomiting, diarrhea)
Progressive Stage (Days 7–14):
- Rash development (maculopapular)
- Conjunctival injection
- Hemorrhagic manifestations: petechiae, ecchymoses, mucosal bleeding
- Shock and hypotension due to vascular leakage
Terminal Stage:
- Multi-organ failure
- Seizures and coma
- Death typically occurs 7 to 14 days after symptom onset without intensive care
Diagnostic Modalities for Confirming Zaire Ebolavirus Infection
Early and accurate diagnosis is essential for containment and treatment.
Laboratory Tests:
- RT-PCR: Gold standard for detecting viral RNA in blood or tissue samples.
- Antigen-capture ELISA: Detects viral proteins in patient serum.
- Virus isolation: Performed in biosafety level 4 (BSL-4) laboratories.
- Serology: Detection of IgM and IgG antibodies; useful in later stages or convalescence.
Supportive Laboratory Findings:
- Thrombocytopenia
- Elevated liver enzymes (AST, ALT)
- Prolonged coagulation times (PT, aPTT)
- Leukopenia followed by leukocytosis
Management Strategies and Treatment Approaches
Currently, there is no universally approved cure, but advances have been made in supportive care and therapeutics.
Supportive Care Includes:
- Fluid and electrolyte replacement
- Oxygen therapy and hemodynamic support
- Correction of coagulation abnormalities
- Symptomatic treatment of pain and fever
Antiviral Therapies and Vaccines:
- Monoclonal antibodies (e.g., Inmazeb, Ebanga): FDA-approved for treatment, targeting the viral glycoprotein.
- Remdesivir: Investigational antiviral with some efficacy.
- Vaccines: rVSV-ZEBOV (Ervebo) vaccine has shown effectiveness in preventing infection during outbreaks.
Infection Control and Prevention Measures
Containment requires strict adherence to infection control protocols:
- Use of personal protective equipment (PPE)
- Isolation of suspected and confirmed patients
- Safe burial practices to prevent transmission from deceased
- Contact tracing and quarantine of exposed individuals
- Public education on avoiding contact with wildlife reservoirs
Public Health Impact and Outbreak Response
Zaire ebolavirus outbreaks demand coordinated international response due to rapid transmission and high mortality.
Key Elements in Outbreak Management:
- Rapid case identification and isolation
- Mobilization of health resources and trained personnel
- Deployment of vaccines in ring vaccination strategies
- Community engagement to reduce stigma and improve compliance
Zaire ebolavirus infection remains a critical global health threat due to its high virulence, rapid progression, and zoonotic potential. Early recognition, prompt supportive care, and novel antiviral interventions are pivotal to improving outcomes. Continued surveillance, vaccine deployment, and public health education are essential to mitigate future outbreaks.