Xerostomia secondary to radiation therapy refers to persistent dry mouth resulting from damage to salivary glands caused by therapeutic ionizing radiation, particularly during head and neck cancer treatment. This condition arises due to radiation-induced salivary gland hypofunction, leading to reduced saliva output and significant impairment in oral and systemic health.
Patients undergoing radiotherapy often experience irreversible salivary gland damage depending on dose, fractionation, and target location. The parotid, submandibular, and minor salivary glands are highly radiosensitive, with long-term effects frequently leading to chronic xerostomia, mucosal discomfort, impaired mastication, dysphagia, and dental decay.
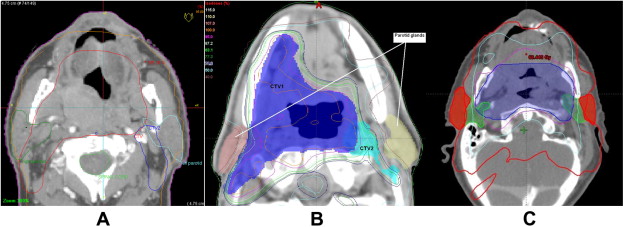
Pathophysiology of Radiation-Induced Salivary Gland Injury
Radiation impacts salivary tissue through:
- Direct cytotoxic effects on serous acinar cells
- Vascular injury reducing perfusion
- Fibrosis and atrophy of glandular stroma
- Oxidative stress damaging DNA and cellular organelles
The parotid glands, which secrete serous, watery saliva, are most affected, leading to an abrupt decrease in unstimulated and stimulated salivary flow, especially within the first two weeks of radiation exposure.
Clinical Manifestations of Xerostomia Post-Radiation
Oral Symptoms
- Constant dry mouth sensation
- Dysphagia, especially with dry or solid foods
- Thick, ropy saliva or complete dryness
- Speech difficulties and hoarseness
- Burning sensation or mucositis
- Loss of taste (ageusia) or altered taste (dysgeusia)
Oral Health Complications
- Rampant dental caries, particularly cervical
- Oral candidiasis
- Halitosis
- Gingivitis and periodontitis
- Difficulty in retaining dentures
Diagnostic Approach to Radiation-Induced Xerostomia
Accurate diagnosis involves quantitative and qualitative assessment of salivary function:
1. Clinical History
- Cancer type, radiation dose (>26 Gy) to salivary glands
- Duration since treatment
- Co-administration of chemotherapy
2. Sialometry
- Unstimulated whole saliva flow < 0.1 mL/min
- Stimulated salivary flow < 0.5 mL/min
3. Imaging Techniques
- Sialography to assess ductal architecture
- Scintigraphy using technetium-99m for glandular function
- MRI/CT for anatomical evaluation in complex cases
Management Strategies for Xerostomia Secondary to Radiation Therapy
Treatment involves symptom relief, salivary function stimulation, and long-term oral preservation.
1. Preventive Measures During Radiotherapy
- Intensity-Modulated Radiation Therapy (IMRT): Precise targeting minimizes exposure to salivary glands
- Salivary Gland-Sparing Techniques: Parotid-sparing protocols reduce xerostomia risk
2. Pharmacologic Stimulation
Pilocarpine
- Cholinergic agonist stimulating residual salivary tissue
- Dosage: 5 mg TID
- Side effects: sweating, flushing, urinary frequency
Cevimeline
- Selective M3 muscarinic receptor agonist
- Fewer CNS side effects than pilocarpine
Note: Effective only if some functional glandular tissue remains.
3. Non-Pharmacological Therapies
- Acupuncture: Enhances parasympathetic stimulation
- Electrostimulation devices: Emerging evidence supports efficacy
- Low-Level Laser Therapy (LLLT): Reduces inflammation and fibrosis
4. Artificial Saliva and Moisturizers
- Carboxymethylcellulose-based rinses and gels
- Sprays and oral lubricants for temporary relief
- Humidifiers for nighttime symptom control
Oral Health Maintenance in Post-Radiation Xerostomia
Fluoride Therapy
- Custom trays with 1.1% sodium fluoride gel
- Daily use prevents demineralization and root caries
Dental Hygiene
- Gentle brushing with soft-bristle brushes
- Non-alcoholic mouthwashes
- Frequent dental checkups, every 3 months
Antifungal and Antibacterial Agents
- Topical nystatin or clotrimazole for candidiasis
- Chlorhexidine gluconate mouth rinses for bacterial control
Nutritional and Lifestyle Recommendations
- Frequent sips of water, avoid sugary or acidic beverages
- Sugar-free gum or lozenges containing xylitol
- Moist soft foods, avoid spicy or dry textures
- Smoking cessation and limit caffeine/alcohol intake
Emerging Therapies and Future Directions
Salivary Gland Regeneration
- Stem cell therapy to regenerate salivary epithelial cells
- Gene therapy using aquaporin-1 gene vectors to enhance water channel formation
Glandular Transposition
- Submandibular gland transfer to submental region pre-radiotherapy
- Protects against irradiation while preserving function
Clinical Trials and Biomarker Research
- Investigating predictive biomarkers for radiation susceptibility
- Proteomics to guide personalized treatment
Quality of Life and Psychosocial Implications
Xerostomia following radiotherapy leads to:
- Social withdrawal due to speaking difficulties and halitosis
- Sleep disturbances
- Depression and anxiety related to chronic discomfort
Integrated support from oncology, dental, speech therapy, and psychological teams ensures holistic patient management.
Frequently Asked Questions:
Q1: How soon does xerostomia develop after radiation therapy?
Dry mouth symptoms can begin within 1–2 weeks of starting radiation and often worsen with cumulative doses.
Q2: Is xerostomia permanent after radiotherapy?
It can be permanent, especially with doses >40 Gy to salivary glands. Recovery is limited in most cases.
Q3: Can pilocarpine reverse xerostomia completely?
No. It stimulates existing salivary tissue but does not regenerate damaged glands.
Q4: What’s the best preventive strategy during cancer treatment?
IMRT, salivary gland shielding, and pre-treatment dental planning are crucial.
Q5: Does xerostomia increase cancer recurrence risk?
No direct correlation, but poor oral hygiene due to dry mouth may lead to secondary infections and complications.
Xerostomia secondary to radiation therapy presents a complex challenge affecting oral health, nutrition, and patient well-being. Early identification, multidisciplinary interventions, and incorporation of advanced therapies can significantly reduce long-term morbidity. As cancer survival improves, optimizing quality of life through effective xerostomia management becomes a crucial aspect of comprehensive oncologic care.