Wiskott-Aldrich Syndrome (WAS) is a rare, X-linked recessive primary immunodeficiency disorder characterized by a clinical triad of eczema, thrombocytopenia with small platelets, and recurrent infections due to combined immunodeficiency. The syndrome is caused by mutations in the WAS gene, which encodes the Wiskott-Aldrich Syndrome protein (WASp), essential for the normal functioning of blood cells, particularly T cells, B cells, and platelets.
Genetic Cause and Pathophysiology
Mutation in the WAS Gene
The WAS gene is located on the X chromosome (Xp11.22–p11.23), and mutations result in either absent or dysfunctional WAS protein. Since the condition is X-linked, it primarily affects males, while females can be carriers.
Function of WASp and Disease Mechanism
WASp is crucial in actin cytoskeleton remodeling, which is vital for cell signaling, movement, immune synapse formation, and phagocytosis. A dysfunctional or missing WASp protein disrupts these cellular processes, resulting in impaired immune responses, abnormal platelet development, and increased susceptibility to infections.
Clinical Manifestations of Wiskott-Aldrich Syndrome
Hematological Symptoms
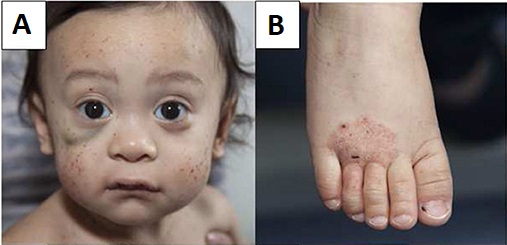
- Thrombocytopenia: Platelet counts are typically <70,000/μL, with platelets markedly smaller than normal. Patients present with petechiae, purpura, epistaxis, gastrointestinal bleeding, or intracranial hemorrhage.
Dermatologic Findings
- Eczema: Often indistinguishable from atopic dermatitis, eczema is typically present from infancy and may be severe and persistent.
Immunologic Complications
- Recurrent Infections: Due to combined T-cell and B-cell immunodeficiency, patients frequently develop otitis media, pneumonia, and sepsis.
- Opportunistic Infections: Viral (e.g., CMV, EBV), fungal, and bacterial infections are common.
Autoimmunity and Malignancies
- Approximately 40-70% of patients develop autoimmune complications such as hemolytic anemia, vasculitis, or inflammatory bowel disease.
- Patients are at increased risk for hematologic malignancies, particularly non-Hodgkin lymphoma.
Diagnostic Evaluation
Clinical Assessment
Diagnosis begins with recognition of the characteristic triad: eczema, bleeding due to thrombocytopenia, and recurrent infections, especially in male infants.
Laboratory Tests
- Complete Blood Count (CBC): Thrombocytopenia with small platelet volume
- Immunoglobulin Profile:
- ↓ IgM
- ↑ IgA and IgE
- Normal or ↑ IgG
- Lymphocyte Subset Analysis: T- and B-cell abnormalities
- Flow Cytometry: Detection of WASp expression in lymphocytes
Genetic Testing
Definitive diagnosis is established by sequencing the WAS gene to identify mutations.
Classification of WAS-Related Disorders
- Classic WAS: Full triad with life-threatening symptoms
- X-linked Thrombocytopenia (XLT): Milder phenotype with predominant bleeding and minimal immunodeficiency
- X-linked Neutropenia: Rare variant with neutrophil dysfunction
Differential Diagnosis
Wiskott-Aldrich Syndrome should be differentiated from:
- Atopic dermatitis (isolated eczema)
- Immune thrombocytopenic purpura (ITP)
- Severe combined immunodeficiency (SCID)
- Hyper IgE syndrome
- Common variable immunodeficiency (CVID)
Management and Treatment Strategies
Hematopoietic Stem Cell Transplantation (HSCT)
HSCT is the only curative treatment for Wiskott-Aldrich Syndrome. Early transplantation, ideally before the onset of severe infections or autoimmunity, significantly improves survival outcomes.
Supportive Care
- IVIG therapy: Monthly infusions to prevent infections
- Antibiotic prophylaxis: Especially against pneumococcus and H. influenzae
- Platelet transfusions: For acute bleeding episodes
- Management of eczema: Topical corticosteroids and skin care
Emerging Therapies: Gene Therapy
Gene therapy using autologous hematopoietic stem cells corrected with a functional WAS gene is under active investigation and has shown promising early results, with successful WASp expression and immune reconstitution.
Prognosis and Life Expectancy
Prognosis largely depends on the severity of the disease and the availability of curative treatment. Without HSCT, life expectancy is significantly reduced due to bleeding complications, infections, and malignancies. With HSCT, especially from a matched sibling donor, the survival rate exceeds 90%.
Genetic Counseling and Carrier Testing
As an X-linked disorder, carrier testing is essential for female relatives of affected individuals. Prenatal diagnosis through chorionic villus sampling or amniocentesis is available for families with known mutations.
Frequently Asked Questions:
Q1: What is the life expectancy for someone with Wiskott-Aldrich Syndrome?
With early HSCT, patients can live into adulthood with normal immune function. Without curative therapy, life expectancy is significantly shortened.
Q2: Can females be affected by WAS?
Because it is X-linked recessive, females are typically carriers. However, skewed X-inactivation may cause mild symptoms in some carriers.
Q3: Is Wiskott-Aldrich Syndrome curable?
Yes, hematopoietic stem cell transplantation is curative in most cases if performed early.
Q4: How is Wiskott-Aldrich Syndrome diagnosed?
Diagnosis involves clinical presentation, immunologic testing, and confirmation by genetic analysis of the WAS gene.
Q5: What causes the bleeding problems in WAS?
Thrombocytopenia with small, dysfunctional platelets results in defective clot formation and spontaneous bleeding.

