Colorectal cancer (CRC) represents a significant global health burden, ranking among the top three most commonly diagnosed cancers worldwide. Among its molecular determinants, RAS oncogenes, particularly KRAS and NRAS, play a pivotal role in tumor behavior and treatment responsiveness. Patients with wild-type KRAS and NRAS colorectal cancer, which denotes the absence of mutations in these genes, exhibit distinct clinical and therapeutic profiles, especially regarding eligibility for epidermal growth factor receptor (EGFR)-targeted therapies.
Understanding RAS Gene Function and Mutational Status
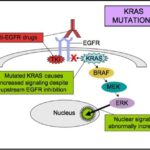
The RAS gene family encodes small GTP-binding proteins involved in the MAPK/ERK signaling pathway, which regulates cell proliferation, differentiation, and survival. In CRC, mutations in KRAS (codons 12, 13, 61, and others) and NRAS disrupt the pathway’s regulation, leading to uncontrolled cellular proliferation.
Wild-Type Definition
- Wild-type KRAS and NRAS means no activating mutations are present in tested exons of these genes.
- These tumors retain the ability to respond to anti-EGFR monoclonal antibodies such as cetuximab and panitumumab.
Prevalence of Wild-Type KRAS and NRAS in CRC
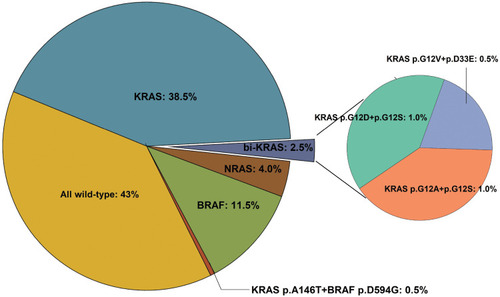
Approximately 40–50% of metastatic colorectal cancer (mCRC) patients have wild-type KRAS. Of those, 80–85% also lack NRAS mutations, classifying them as pan-RAS wild-type.
- Wild-type RAS is more common in:
- Left-sided colon cancers
- Lower tumor grade
- Earlier-stage disease
These molecular profiles offer predictive value for both therapeutic response and disease progression.
Importance of Extended RAS Testing in CRC Management
Current clinical guidelines (e.g., NCCN, ESMO, ASCO) mandate extended RAS testing prior to initiating EGFR-targeted therapy in metastatic CRC. This includes KRAS exons 2, 3, and 4 and NRAS exons 2, 3, and 4.
Testing Methods
- PCR-based assays
- Next-generation sequencing (NGS)
- Digital droplet PCR (ddPCR)
These platforms ensure high sensitivity in detecting even low-frequency mutations, allowing for precise treatment selection.
EGFR Inhibitor Therapy in Wild-Type KRAS and NRAS CRC
Patients with confirmed wild-type KRAS and NRAS status are eligible for anti-EGFR therapy, which is ineffective in mutated RAS tumors due to intrinsic resistance.
Key Agents
- Cetuximab: Chimeric monoclonal antibody targeting EGFR
- Panitumumab: Fully human monoclonal antibody with similar efficacy
These agents are typically used in:
- First-line treatment in combination with chemotherapy (FOLFIRI or FOLFOX)
- Second-line monotherapy
- Maintenance settings
Clinical Outcomes
- Improved progression-free survival (PFS)
- Increased overall survival (OS)
- Enhanced tumor response rate (RR)
Left-sided tumors respond more favorably to EGFR blockade than right-sided ones, even within wild-type RAS cohorts.
Biomarkers Beyond KRAS and NRAS: BRAF, MSI, and HER2
While KRAS and NRAS are foundational for EGFR therapy eligibility, additional biomarkers can refine treatment strategies further.
| Biomarker | Mutation Status | Clinical Impact |
|---|---|---|
| BRAF V600E | Often co-mutated with wild-type RAS | Poor prognosis, EGFR therapy resistance |
| MSI-High/dMMR | Associated with immune checkpoint therapy response | May predict poor EGFR response |
| HER2 Amplification | Wild-type RAS context | Targetable with HER2 inhibitors |
For optimal outcomes, multi-biomarker profiling is essential.
Prognostic Implications of Wild-Type RAS in CRC
Patients with wild-type KRAS and NRAS tumors generally have:
- Better prognosis
- More therapeutic options
- Slower progression compared to RAS-mutated counterparts
These patients benefit from personalized, targeted therapies and are often considered for clinical trials exploring next-generation biologics or combination regimens.
Emerging Research and Future Directions
Resistance Mechanisms
Even in wild-type KRAS/NRAS tumors, acquired resistance to EGFR therapy can develop. Mechanisms include:
- Secondary RAS mutations
- EGFR extracellular domain alterations
- Amplification of alternative pathways (e.g., MET, HER2)
Liquid Biopsies
The advent of circulating tumor DNA (ctDNA) testing enables non-invasive monitoring for emerging mutations and therapy adaptation in real time.
Immunotherapy Combinations
Ongoing trials are evaluating EGFR inhibitors with checkpoint inhibitors or targeted kinase inhibitors in biomarker-enriched populations.
Wild-type KRAS and NRAS colorectal cancer represents a distinct clinical entity with favorable therapeutic responsiveness to EGFR-targeted strategies. Comprehensive RAS profiling, alongside broader molecular characterization, is now a cornerstone of personalized CRC management. With continued advances in molecular diagnostics, biologic therapies, and precision oncology, outcomes for this subgroup of patients continue to improve.