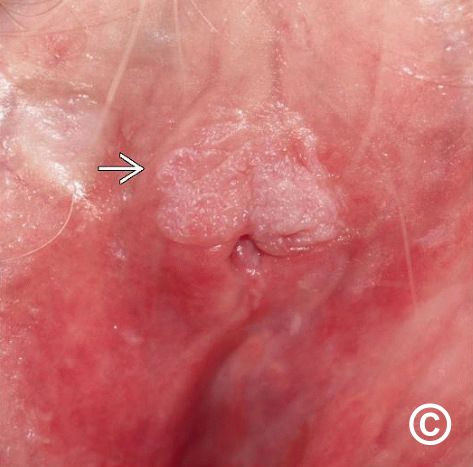
Vulvar intraepithelial neoplasia (VIN) represents a spectrum of precancerous lesions involving the squamous epithelium of the vulva. These changes, although non-invasive, carry the potential to progress into invasive vulvar carcinoma if left undiagnosed or untreated. The clinical relevance of VIN has grown due to its association with human papillomavirus (HPV), particularly HPV-16, and its increasing prevalence among younger and immunocompromised women.
Classification of VIN: Differentiating Subtypes
VIN is categorized based on histopathological features and etiological factors. The International Society for the Study of Vulvovaginal Disease (ISSVD) defines two main subtypes:
1. Usual-Type VIN (uVIN)
- Strongly associated with high-risk HPV infection (especially HPV-16)
- Common in younger women (ages 20–50)
- Subtypes: warty, basaloid, and mixed
- Risk factors: smoking, multiple sexual partners, immunosuppression
2. Differentiated-Type VIN (dVIN)
- Not HPV-related
- Typically affects postmenopausal women
- Frequently associated with chronic dermatoses such as lichen sclerosus
- Higher potential for rapid progression to invasive carcinoma
Causes and Risk Factors of VIN
The etiology of VIN varies between the subtypes:
- High-Risk HPV Infection: The most significant factor in uVIN; persistent infection can lead to dysplastic changes.
- Chronic Inflammatory Dermatoses: Especially lichen sclerosus, commonly seen in dVIN.
- Immunosuppression: Patients with HIV or those on immunosuppressive therapy have a heightened risk.
- Smoking: Facilitates HPV persistence and impairs epithelial healing.
- Sexual Behavior: Early sexual debut and multiple sexual partners increase the risk of HPV exposure.
Recognizing the Symptoms and Clinical Presentation
VIN can be asymptomatic in its early stages, which contributes to delayed diagnosis. When symptoms do occur, they are often nonspecific.
Common Symptoms:
- Persistent vulvar itching or burning
- Pain during intercourse (dyspareunia)
- Notable changes in vulvar skin (discoloration, thickening, or ulceration)
- Palpable or visible lesions (raised, warty, or plaque-like)
Lesions are most commonly located on the labia majora, labia minora, and perineal region, and may be multifocal.
Diagnostic Evaluation of VIN
Accurate diagnosis is critical for effective treatment and long-term monitoring. Clinical suspicion should prompt thorough evaluation.
Diagnostic Procedures:
- Visual Inspection: Under magnification with colposcopy or a vulvoscope
- Acetic Acid Application: Highlights abnormal epithelial changes
- Biopsy: Confirms diagnosis and excludes invasive carcinoma
- Histopathology: Determines VIN subtype, grade, and presence of dermatoses
Additional Investigations:
- HPV Typing: Identifies high-risk viral strains
- Immunohistochemistry: p16 and Ki-67 staining to differentiate uVIN from dVIN
- Assessment for Coexisting Conditions: Lichen sclerosus, squamous cell carcinoma in situ
Management and Treatment Options for VIN
The goal of VIN treatment is to eliminate lesions, reduce symptom burden, prevent progression to carcinoma, and preserve vulvar anatomy.
1. Surgical Treatment
- Wide Local Excision: Removes lesion with clear margins
- Skinning Vulvectomy: Reserved for extensive multifocal disease
- Laser Ablation: Vaporizes superficial lesions without tissue removal
- Consideration: High recurrence rate with incomplete excision or untreated HPV
2. Medical Therapy
- Topical Imiquimod (5%)
- Immune response modifier
- Suitable for multifocal, non-invasive uVIN
- Course: 2–3 times weekly for up to 16 weeks
- Side effects: erythema, irritation, pain
- Cidofovir Gel: Antiviral agent under investigation for refractory cases
- 5-Fluorouracil (5-FU): Less commonly used due to high irritation potential
3. Photodynamic Therapy (PDT)
- Utilizes photosensitizing agents activated by light
- Minimally invasive
- Limited by availability and variable efficacy
Follow-Up and Surveillance
VIN requires long-term monitoring due to the risk of recurrence and malignant transformation, especially in dVIN.
Surveillance Protocol:
- Initial Post-Treatment Follow-Up: Every 3–6 months for the first 2 years
- Annual Exams: Thereafter for at least 5 years
- Biopsy of New Lesions: Prompt histological reassessment for recurrent or progressive disease
- Counseling and HPV Vaccination: Offer to women with uVIN and their partners
Prevention Strategies
HPV Vaccination
- Highly effective in preventing HPV-associated VIN and cervical neoplasia
- Vaccines Available:
- Gardasil 9 (targets HPV-6, -11, -16, -18 and others)
- Administered in adolescents and young adults before sexual debut
Smoking Cessation
- Enhances immune response and lesion regression
- Decreases recurrence risk
Management of Dermatologic Disorders
- Prompt treatment of lichen sclerosus reduces dVIN risk
- Regular follow-up with dermatology and gynecology is essential
Complications and Prognosis
Complications:
- Vulvar disfigurement or scarring from surgery
- Psychosexual dysfunction
- Chronic pain or dyspareunia
Prognosis:
- uVIN: Generally favorable with appropriate treatment
- dVIN: Higher recurrence and rapid progression to invasive squamous cell carcinoma
- Recurrence Rates: Up to 30–50% in high-risk cases or incomplete treatment
Patient Education and Support
Educating patients about VIN is vital to ensuring compliance and early intervention. Supportive resources include:
- Counseling for sexual health and anxiety
- Support groups for gynecologic precancers
- Guidance on lifestyle changes such as smoking cessation and safe sexual practices
Vulvar intraepithelial neoplasia is a significant precancerous condition that requires early recognition, accurate diagnosis, and tailored treatment. With increasing awareness, access to HPV vaccination, and advanced diagnostic tools, the progression to invasive vulvar carcinoma can be effectively minimized. Continuous surveillance and patient-centered care remain essential to improving outcomes and quality of life for those affected by VIN.