Vaginal cancer is a rare but serious malignancy of the female reproductive tract. A significant proportion of these cases are linked to persistent infection with high-risk human papillomavirus (HPV) types, most notably HPV 16 and 18. These oncogenic strains are known to cause cellular changes in the vaginal epithelium, leading to vaginal intraepithelial neoplasia (VaIN), which can progress to invasive carcinoma if left untreated.
HPV-associated vaginal cancers often occur in older women, but HPV infection typically occurs decades earlier, underscoring the importance of early vaccination before viral exposure.
Human Papillomavirus: A Causal Agent of Vaginal Malignancy
HPV is a non-enveloped, double-stranded DNA virus with more than 200 genotypes. These genotypes are classified as:
- Low-risk types (e.g., HPV 6 and 11): cause genital warts.
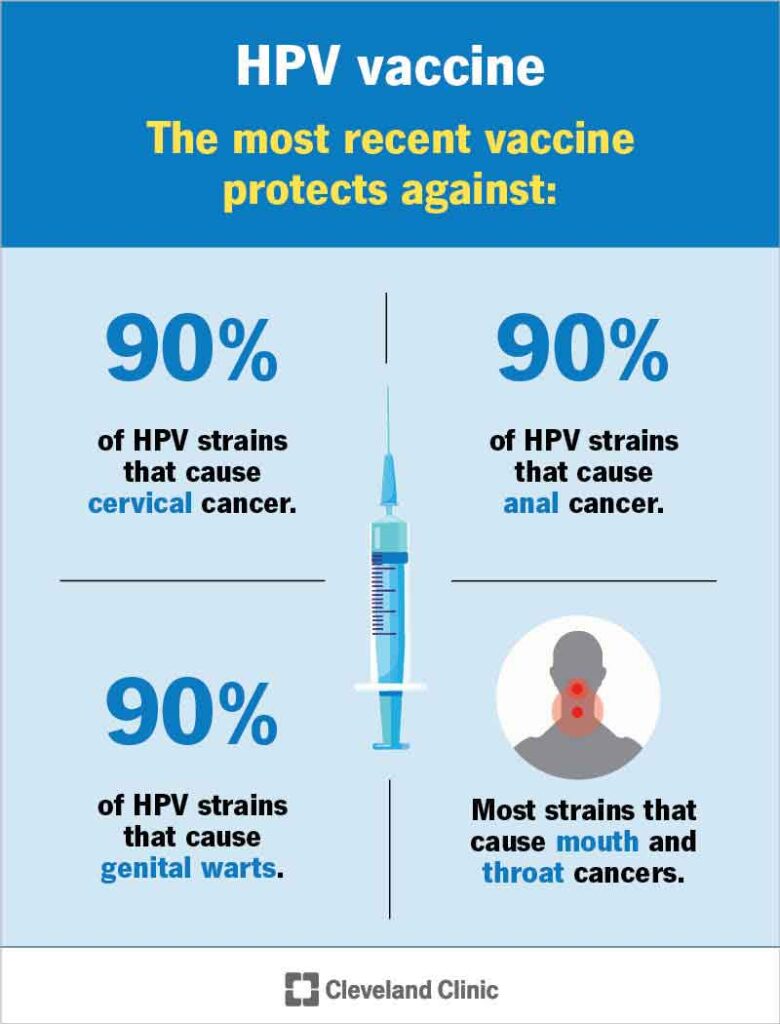
- High-risk types (e.g., HPV 16, 18, 31, 33, 45): associated with various cancers, including vaginal, cervical, vulvar, anal, and oropharyngeal cancers.
Persistent infection with high-risk HPV can lead to oncogenic transformation through integration of viral DNA into host cells, disrupting tumor suppressor genes such as p53 and Rb.
HPV Vaccination: A Preventive Tool Against Vaginal Cancer
How the Vaccine Works
HPV vaccines are composed of virus-like particles (VLPs) formed from the L1 capsid protein of the virus. These VLPs are non-infectious but highly immunogenic, provoking a strong antibody response that prevents initial HPV infection and cellular entry.
By blocking infection from high-risk HPV types, the vaccine prevents the precursor lesions (VaIN 2/3) that lead to vaginal cancer.
Available HPV Vaccines and Their Efficacy
Three HPV vaccines have been developed and approved for clinical use. Among these, Gardasil 9 is the most comprehensive.
| Vaccine | HPV Types Covered | Approved For | Cancer Protection |
|---|---|---|---|
| Gardasil 4 | 6, 11, 16, 18 | Males and females (9–26 yrs) | Cervical, vaginal, vulvar, and anal cancer |
| Gardasil 9 | 6, 11, 16, 18, 31, 33, 45, 52, 58 | Males and females (9–45 yrs) | Broader HPV cancer prevention including vaginal cancer |
| Cervarix | 16, 18 | Females (10–25 yrs) | Cervical and vaginal cancer |
Gardasil 9 offers protection against the widest array of high-risk HPV types and is currently the preferred vaccine in most national immunization programs.
Clinical Evidence Supporting HPV Vaccine in Vaginal Cancer Prevention
Multiple clinical trials have demonstrated the high efficacy of HPV vaccination in preventing HPV-related vaginal lesions.
- FUTURE II Trial: Showed Gardasil significantly reduced the incidence of VaIN 2/3 caused by HPV 16 and 18.
- Real-world data from countries with high vaccine coverage show a decline in HPV prevalence and pre-cancerous vaginal lesions in young women.
A meta-analysis published in The Lancet confirmed a 90% reduction in HPV 16/18 infections and associated lesions in vaccinated populations under the age of 25.
Recommended Vaccination Schedule
Routine Vaccination Schedule
| Age Group | Dosing Schedule |
|---|---|
| 9–14 years | 2 doses (0 and 6–12 months) |
| 15–26 years | 3 doses (0, 1–2 months, and 6 months) |
| 27–45 years* | 3 doses (based on shared clinical decision-making) |
*For individuals up to 45 years, the vaccine is most beneficial if they are not previously exposed to HPV.
Timing and Target Population
Vaccination is most effective when administered before the onset of sexual activity, typically between the ages of 9 and 12. However, catch-up vaccination is beneficial up to age 26, and selectively for adults aged 27–45.
Immunocompromised individuals, including those with HIV, are advised to receive a three-dose schedule regardless of age.
Public Health Impact and Global Vaccination Programs
HPV vaccination programs have been integrated into national immunization schedules in over 100 countries. Benefits include:
- Reduced incidence of vaginal intraepithelial neoplasia
- Decline in HPV prevalence in sexually active populations
- Long-term prevention of vaginal, cervical, and vulvar cancers
In Australia, one of the earliest adopters of school-based HPV immunization, precancerous vaginal lesions have significantly declined since vaccine rollout.
Safety and Side Effects of HPV Vaccines
HPV vaccines have been extensively studied and deemed safe and well-tolerated by global health authorities, including WHO and CDC.
Common Side Effects:
- Local site pain, redness, or swelling
- Mild fever
- Fatigue or headache
Rare Events:
- Anaphylaxis (extremely rare)
- Syncope (managed by observing patients for 15 minutes post-vaccination)
No causal association has been found between HPV vaccination and fertility issues or autoimmune disorders.
Addressing Vaccine Hesitancy and Myths
Despite proven efficacy, vaccine uptake is hindered in some regions due to misinformation. Education efforts should focus on:
- Emphasizing cancer prevention benefits
- Highlighting extensive safety data
- Clarifying that the vaccine is not a license for early sexual activity, but a proactive health measure
Parental consent programs and healthcare provider advocacy are key drivers of successful HPV vaccination implementation.
Vaccination against human papillomavirus represents the most powerful tool in preventing vaginal cancer and its precancerous stages. With strong scientific backing, a favorable safety profile, and increasing global adoption, HPV vaccines are reshaping the landscape of gynecologic cancer prevention.
Widespread administration of Gardasil 9, particularly prior to sexual debut, ensures long-term protection against high-risk HPV types, safeguarding the reproductive health of future generations.