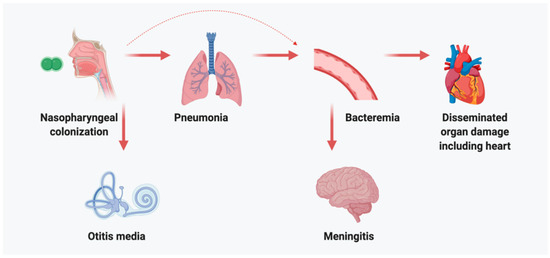
Otitis media is one of the most prevalent pediatric infections globally, characterized by inflammation and fluid accumulation in the middle ear. The condition manifests through ear pain, fever, and sometimes hearing loss, often requiring medical intervention.
Among its primary bacterial causes, Streptococcus pneumoniae—a gram-positive diplococcus—is responsible for a significant portion of acute otitis media (AOM) cases in infants and young children. This pathogen colonizes the nasopharynx and ascends the eustachian tube to infect the middle ear, triggering inflammatory responses and mucosal effusion.
Pneumococcal Conjugate Vaccines: A Cornerstone in Otitis Media Prevention
The introduction of pneumococcal conjugate vaccines (PCVs), particularly PCV7, PCV10, and PCV13, has revolutionized the prevention of diseases associated with S. pneumoniae, including otitis media.
Mechanism of Protection
PCVs contain capsular polysaccharides of selected pneumococcal serotypes conjugated to a carrier protein (e.g., diphtheria CRM197). This conjugation enables:
- T-cell–dependent immune response
- Long-term immunological memory
- Effective mucosal immunity that limits nasopharyngeal colonization
By reducing the reservoir of S. pneumoniae in the upper respiratory tract, PCVs lower the incidence of bacterial otitis media and reduce transmission within communities.
Key Pneumococcal Vaccines Used in Otitis Media Prevention
| Vaccine | Serotypes Covered | Target Population | Protection Against Otitis Media |
|---|---|---|---|
| PCV7 | 4, 6B, 9V, 14, 18C, 19F, 23F | Infants and toddlers | 20–30% reduction in AOM |
| PCV10 | PCV7 + 1, 5, 7F | Broader pediatric coverage | 35–45% AOM reduction |
| PCV13 | PCV10 + 3, 6A, 19A | Expanded serotype coverage | Up to 50% AOM reduction |
The PCV13 vaccine is currently the most comprehensive in terms of serotype inclusion and impact on otitis media prevention.
Vaccine Effectiveness in Reducing Otitis Media Incidence
Multiple large-scale studies confirm the significant decline in otitis media cases following routine PCV implementation:
- United States (post-PCV7 era): A 39% reduction in physician visits for otitis media in children under 2 years.
- Finland (PCV10 program): 25% decrease in AOM episodes requiring antibiotics.
- Australia (PCV13 introduction): Hospitalizations for otitis media dropped by over 50% in vaccinated cohorts.
Vaccination also contributes to reduced antibiotic prescriptions, mitigating antimicrobial resistance associated with otitis media management.
Recommended Immunization Schedule for Pneumococcal Vaccines
Vaccination timing is critical for optimal immunity, particularly during the highest risk period in infancy.
Infant and Toddler Dosing (Routine Schedule):
| Age | Dose |
|---|---|
| 2 months | First dose (PCV13) |
| 4 months | Second dose |
| 6 months | Third dose |
| 12–15 months | Booster dose |
Catch-Up Schedules
Children aged 7 months to 5 years who are unvaccinated or under-vaccinated should follow age-appropriate catch-up schedules, ensuring full serotype coverage.
Public Health Impact of Pneumococcal Vaccination
The implementation of PCV programs yields substantial public health benefits:
- Herd Immunity: Unvaccinated individuals benefit from reduced circulation of vaccine-covered serotypes.
- Healthcare Cost Reduction: Fewer clinic visits, surgical interventions (e.g., tympanostomy tube insertion), and antibiotic courses.
- Decreased Complications: Lower incidence of serious sequelae like mastoiditis, hearing impairment, and speech delays.
Shifts in Serotype Distribution and Emerging Challenges
While PCVs dramatically reduce disease from vaccine-included serotypes, non-vaccine serotypes (NVTs) have emerged in some regions. This phenomenon, known as serotype replacement, underscores the need for:
- Next-generation PCVs covering additional serotypes
- Ongoing surveillance to track epidemiological shifts
- Alternative vaccine strategies, including protein-based or whole-cell vaccines under development
Nevertheless, the net effect of PCVs remains strongly positive in preventing otitis media due to S. pneumoniae.
Integration With Broader Pediatric Immunization Programs
PCVs are routinely co-administered with other childhood vaccines such as DTaP, Hib, and rotavirus, maximizing immunization coverage and adherence. Their inclusion in national immunization schedules across over 150 countries emphasizes their global priority status.
Effective implementation depends on:
- Healthcare provider advocacy
- Parental education on vaccine benefits
- Sustained government funding and accessibility
Otitis media caused by Streptococcus pneumoniae represents a major health concern for infants and children worldwide. The pneumococcal conjugate vaccine, particularly PCV13, offers a scientifically validated, widely endorsed, and highly effective intervention to prevent this condition.
Vaccination during early childhood not only safeguards against acute infections but also contributes to long-term health by reducing recurrent episodes, hearing loss, and antibiotic overuse. As new vaccine formulations evolve, continued investment in pneumococcal immunization strategies remains imperative to protecting young populations and strengthening public health resilience.