Unresectable or metastatic KIT (CD117) positive gastrointestinal stromal tumors (GISTs) represent a distinct subset of mesenchymal neoplasms that originate primarily in the gastrointestinal tract. These tumors are characterized by activating mutations in the KIT gene, which encodes a receptor tyrosine kinase that drives tumor proliferation. The advent of targeted therapies has transformed the management of advanced GIST, offering extended survival and improved quality of life.
Understanding KIT (CD117) Positive Gastrointestinal Stromal Tumors
GISTs account for approximately 1–2% of all gastrointestinal malignancies. Most arise from the interstitial cells of Cajal and exhibit overexpression or mutation of the KIT (CD117) receptor tyrosine kinase.
Key Features
- Origin: Predominantly in the stomach (60%) and small intestine (30%)
- Molecular hallmark: Gain-of-function mutations in KIT (exons 9, 11, 13, 17) or PDGFRA
- Immunohistochemistry: Strong positivity for CD117 and DOG1
- Clinical Presentation: GI bleeding, abdominal pain, palpable mass, or incidental findings
Defining Unresectable and Metastatic GIST
Unresectable GIST
GISTs are deemed unresectable when:
- Involvement of critical structures prevents safe resection
- Multiple intra-abdominal lesions preclude curative surgery
- Complete resection would require removal of vital organs
Metastatic GIST
- Common metastatic sites: Liver, peritoneum, and rarely lungs
- Often present at diagnosis or recur after primary tumor resection
- Prognosis varies by mutation subtype and response to therapy
Diagnostic Workup for Advanced GIST
Imaging
- Contrast-enhanced CT: First-line modality to assess tumor size, location, and metastasis
- MRI: Useful for rectal or pelvic GISTs
- PET scan: Evaluates early treatment response
Molecular Testing
Mutation profiling is essential to guide targeted therapy selection:
- KIT exon 11: Most common; excellent response to imatinib
- KIT exon 9: Requires higher imatinib dosing
- PDGFRA D842V: Resistant to imatinib, responsive to avapritinib
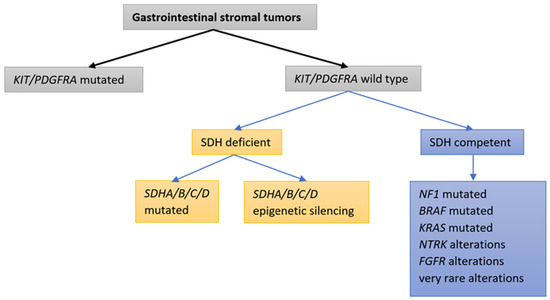
- Wild-type GIST: Associated with alternative pathways (SDH, BRAF)
Treatment of Unresectable or Metastatic KIT (CD117) Positive GIST
First-Line Therapy: Imatinib Mesylate
- Mechanism: Selective inhibition of KIT and PDGFRA tyrosine kinases
- Dosage: 400 mg daily (800 mg for KIT exon 9 mutations)
- Outcomes: Median progression-free survival (PFS) of 18–24 months
Second-Line Therapy: Sunitinib Malate
- Administered upon imatinib resistance or intolerance
- Inhibits KIT, PDGFR, VEGFR
- PFS of 6–9 months; well-tolerated in most patients
Third-Line Therapy: Regorafenib
- Multi-kinase inhibitor effective post-imatinib and sunitinib failure
- Shown to improve PFS in the GRID trial
Fourth-Line Therapy and Beyond
Ripretinib
- Switch-control kinase inhibitor targeting KIT and PDGFRA mutations
- Approved for patients with ≥3 prior lines of therapy
- Prolongs median PFS with manageable toxicity
Avapritinib
- Potent selective inhibitor for PDGFRA D842V mutations
- Also active in some KIT-driven GISTs
- Recommended for mutation-confirmed resistant tumors
Surgical and Interventional Considerations
While systemic therapy is the cornerstone of treatment, surgery may be appropriate in select cases:
- Resection post-response: To reduce tumor burden or treat resistant clones
- Palliative surgery: For bleeding, obstruction, or pain control
- Radiofrequency ablation or embolization: For isolated hepatic metastases
Monitoring and Managing Disease Progression
Imaging Follow-Up
- Every 3–6 months during active treatment
- Monitor for new lesions, size increase, and density change
Managing Resistance
- Secondary KIT mutations drive acquired resistance
- Strategies include switching TKIs, re-biopsy, and combination therapies
- Clinical trials are essential for access to novel agents
Prognosis and Survival Outcomes
Prognostic Factors
- Mutation subtype (KIT exon 11 favorable; exon 9 intermediate; PDGFRA D842V poor)
- Tumor size and mitotic rate
- Response to tyrosine kinase inhibitors
Median Overall Survival
- With targeted therapy: 5–6 years
- Wild-type or resistant subtypes: Reduced survival
- Importance of early molecular profiling and trial access
Future Directions and Clinical Trials
Ongoing research is focused on:
- Next-generation TKIs with broader resistance coverage
- Immunotherapy combinations
- Biomarker-driven personalized treatment
- Targeting alternative pathways in wild-type GISTs (e.g., SDH-deficient tumors)
Participation in clinical trials is highly recommended for patients with refractory disease or rare mutation types.
Management of unresectable or metastatic KIT (CD117) positive gastrointestinal stromal tumors has advanced substantially due to precision-targeted therapies. Early identification of mutation subtype, prompt initiation of appropriate tyrosine kinase inhibitors, and regular monitoring are pivotal to extending survival. Integration of systemic and localized therapies, along with clinical trial enrollment, offers the best strategy to combat this challenging malignancy.