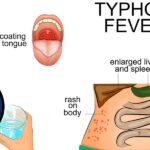
Typhoid fever continues to be a public health challenge in low- and middle-income countries. Vaccination plays a pivotal role in preventing Salmonella enterica serotype Typhi infections, especially in endemic regions and among travelers. A robust understanding of typhoid vaccine types, administration schedules, and effectiveness is essential for healthcare professionals and policy-makers to reduce disease burden and prevent outbreaks.
Understanding Typhoid Fever and the Need for Vaccination
Typhoid fever is a systemic illness transmitted via the fecal-oral route, primarily through contaminated food and water. With an annual global burden exceeding 11 million cases and 100,000 deaths, the disease disproportionately affects children and young adults in regions with poor sanitation.
Vaccination is the most effective tool alongside sanitation and hygiene to reduce transmission. The World Health Organization (WHO) recommends incorporating typhoid vaccines in routine immunization programs in endemic areas.
Types of Typhoid Vaccines and Their Characteristics
There are three primary typhoid vaccines approved for use globally:
1. Vi Polysaccharide Vaccine (Injectable)
- Composition: Purified Vi capsular polysaccharide antigen from S. Typhi.
- Administration: Single intramuscular injection.
- Age eligibility: Approved for individuals ≥2 years of age.
- Booster requirement: Every 2 to 3 years for continued protection.
- Efficacy: ~60–70% efficacy within the first two years.
2. Ty21a Vaccine (Oral Live-Attenuated)
- Composition: Live attenuated strain of S. Typhi.
- Administration: Oral, 3–4 doses on alternate days.
- Age eligibility: For individuals ≥6 years.
- Booster requirement: Every 5–7 years in travelers.
- Efficacy: ~50–80% based on compliance and local epidemiology.
- Special consideration: Should not be used in immunocompromised individuals.
3. Typhoid Conjugate Vaccine (TCV)
- Composition: Vi polysaccharide conjugated to a carrier protein (e.g., tetanus toxoid).
- Administration: Single intramuscular dose.
- Age eligibility: Suitable from 6 months of age.
- Booster requirement: Under evaluation; long-term protection expected.
- Efficacy: >80% with longer-lasting immunity.
- WHO-preferred vaccine: Recommended for national immunization programs.
Typhoid Vaccine Schedule and Dosage Recommendations
For Routine Immunization
- Typhoid Conjugate Vaccine (TCV): One dose at 9–12 months of age, integrated with other childhood vaccines.
- Booster dose: WHO suggests monitoring for booster needs, but current evidence indicates protection for at least 5 years.
For Travelers to Endemic Regions
- Vi Polysaccharide Vaccine: Single injection at least 2 weeks before travel; booster every 2–3 years.
- Ty21a Oral Vaccine: 3–4 doses taken on alternate days; final dose must be completed 1 week before travel.
In Outbreak Situations
- TCV is preferred for rapid immunization of populations in outbreaks, especially in children.
Effectiveness and Duration of Protection
Each vaccine varies in its protective efficacy and duration. TCV has emerged as the superior option due to its:
- Long-term protection,
- Suitability for younger children,
- Induction of T-cell dependent immunity.
Comparative Overview:
| Vaccine Type | Age Eligibility | Doses | Booster Needed | Efficacy |
|---|---|---|---|---|
| Vi Polysaccharide | ≥2 years | 1 IM shot | Every 2–3 yrs | 60–70% |
| Ty21a (Oral) | ≥6 years | 3–4 doses | Every 5–7 yrs | 50–80% |
| TCV | ≥6 months | 1 IM shot | TBD | >80% |
Safety, Contraindications, and Side Effects
Common Side Effects
- Injection site pain or swelling (Vi and TCV)
- Mild fever, headache, or nausea
- Abdominal discomfort (Ty21a)
Contraindications
- Ty21a is contraindicated in immunocompromised individuals and during acute gastrointestinal illness.
- Live vaccines should be avoided in pregnant women unless the benefit outweighs the risk.
All vaccines are generally well-tolerated and safe.
WHO Recommendations and Global Implementation
WHO recommends TCV introduction in endemic countries for children aged 6 months and older. Many nations including India, Pakistan, and several African countries have integrated TCVs into national immunization schedules.
Key WHO Strategies Include:
- Routine immunization with TCV
- Catch-up campaigns for children up to 15 years
- Integration with water and sanitation improvement programs
Integration with Public Health Measures
While vaccination is critical, it should be combined with other interventions:
- Water sanitation: Access to clean drinking water is vital.
- Hygiene education: Hand hygiene, food safety practices.
- Surveillance: Monitoring of typhoid incidence and resistance patterns.
Addressing Antimicrobial Resistance Through Vaccination
The emergence of multidrug-resistant and extensively drug-resistant S. Typhi strains underscores the urgent need for wide-scale vaccination. TCV, by reducing the incidence of typhoid, can significantly diminish reliance on antibiotics and help combat antimicrobial resistance (AMR).
Future Directions and Innovations
- Next-generation vaccines: Multivalent vaccines targeting S. Paratyphi A are under development.
- Single-dose oral formulations: Research is ongoing to improve compliance and shelf-life.
- Global funding: Gavi, the Vaccine Alliance, supports the introduction of TCV in low-income countries.
Typhoid vaccination remains a cornerstone of global strategies to combat enteric fever, especially in regions with limited access to clean water and sanitation. Among the available options, Typhoid Conjugate Vaccine (TCV) provides the most promising solution due to its long-lasting protection, applicability to infants, and WHO endorsement. To achieve substantial reductions in typhoid morbidity and mortality, widespread immunization must be complemented with public health interventions, surveillance, and global cooperation.