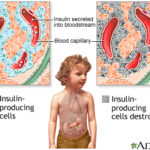
Management of type 1 diabetes mellitus (T1DM) has traditionally relied on exogenous insulin replacement. However, despite intensive insulin therapy and technological advancements, many individuals still fail to achieve optimal glycemic control. Adjunctive therapies are increasingly being explored to complement insulin, aiming to reduce glucose variability, improve HbA1c, minimize insulin requirements, and address comorbidities. This article offers a detailed examination of current and emerging adjunct treatments for T1DM.
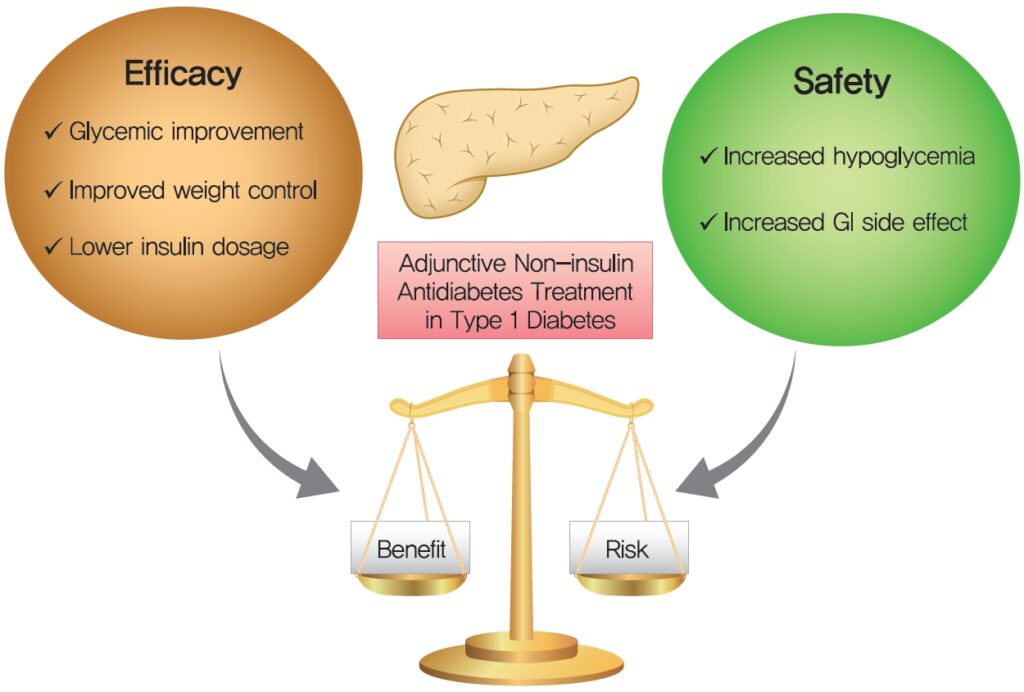
The Rationale for Adjunctive Therapies in Type 1 Diabetes Mellitus
Insulin therapy alone often falls short in addressing the full spectrum of metabolic abnormalities in T1DM. Adjunct treatments provide several benefits:
- Improve postprandial glucose control
- Reduce glycemic variability
- Lower total daily insulin dose
- Promote weight loss or prevent weight gain
- Reduce risk of hypoglycemia
- Address cardiovascular and renal comorbidities
Limitations of Insulin-Only Therapy
| Issue | Implication |
|---|---|
| Risk of hypoglycemia | Limits dose titration |
| Weight gain | Affects adherence and metabolic profile |
| Insulin resistance | Especially in overweight patients |
| Lack of control over postprandial spikes | Increases HbA1c variability |
Pramlintide: A Synthetic Amylin Analog
Pramlintide is the only FDA-approved adjunctive medication specifically indicated for T1DM. It is an analog of amylin, a hormone co-secreted with insulin by pancreatic beta cells.
Mechanism of Action
- Delays gastric emptying
- Suppresses postprandial glucagon secretion
- Enhances satiety
Clinical Benefits
- Improved postprandial glucose control
- Reduced HbA1c (~0.3–0.4%)
- Modest weight loss (~1–2 kg)
- Lower prandial insulin requirements
Considerations
- Injectable, requires separate administration from insulin
- Associated with nausea, particularly during initiation
- Risk of hypoglycemia when insulin is not properly adjusted
SGLT2 Inhibitors: Cardiometabolic Benefits in T1DM
Sodium-glucose co-transporter 2 (SGLT2) inhibitors, while originally developed for type 2 diabetes, have demonstrated benefits as adjuncts in T1DM.
Mechanism of Action
- Promote renal glucose excretion
- Lower plasma glucose levels independent of insulin
Agents Studied in T1DM
- Dapagliflozin
- Empagliflozin
- Sotagliflozin (dual SGLT1/2 inhibitor)
Clinical Evidence
- HbA1c reduction: ~0.3–0.5%
- Weight loss: ~2–3 kg
- Insulin dose reduction: ~10–15%
Safety Concerns
- Euglycemic diabetic ketoacidosis (DKA) is a significant risk
- Not FDA-approved for T1DM due to safety profile
- Requires patient education and ketone monitoring
GLP-1 Receptor Agonists: Emerging Role in Type 1 Diabetes
Glucagon-like peptide-1 (GLP-1) receptor agonists, such as liraglutide and semaglutide, have shown promise in insulin-treated patients with obesity or insulin resistance.
Mechanism of Action
- Slows gastric emptying
- Suppresses glucagon
- Promotes satiety
- Enhances insulin sensitivity
Clinical Outcomes
- HbA1c reduction: modest (~0.3–0.4%)
- Significant weight loss: up to 4–5 kg
- Lower insulin requirement
- Improved metabolic profile
Limitations
- Not approved for T1DM
- Risk of gastrointestinal side effects (nausea, vomiting)
- Risk of hypoglycemia when combined with insulin
Metformin: A Traditional Agent in a New Role
Although typically reserved for type 2 diabetes, metformin may offer limited benefits as an adjunct in overweight T1DM patients.
Benefits in Select T1DM Populations
- Modest reduction in HbA1c
- Reduction in insulin dose (~6–10%)
- Improved insulin sensitivity
- Favorable lipid profile
Caveats
- Limited effect on weight or glycemic variability
- Gastrointestinal discomfort
- Not routinely recommended unless insulin resistance is evident
Adjunctive Therapy Selection: Patient-Centric Approach
Individualizing adjunct therapy based on clinical profile is critical. The following matrix outlines key considerations:
| Patient Profile | Preferred Adjunct | Rationale |
|---|---|---|
| High postprandial glucose | Pramlintide | Targets post-meal glucose excursions |
| Overweight with insulin resistance | GLP-1 RA or SGLT2 inhibitor | Weight loss and insulin-sparing effects |
| Cardiovascular comorbidities | SGLT2 inhibitor | Cardioprotective potential |
| Frequent hypoglycemia | GLP-1 RA (with insulin adjustment) | Reduce variability and insulin burden |
| Insulin-resistant adolescents | Metformin | Enhances insulin sensitivity |
Safety Monitoring and Risk Mitigation
Implementing adjunct therapies necessitates careful safety protocols:
- Ketone monitoring: Essential for patients on SGLT2 inhibitors
- Education: Patients must understand risks of hypoglycemia and DKA
- Dosing adjustments: Insulin doses must be recalibrated
- Follow-up: Regular evaluation of renal function, electrolytes, and weight
Future Directions in Adjunctive Therapies for T1DM
Ongoing research continues to explore innovative therapeutic avenues:
Immunomodulatory Therapies
- Agents like teplizumab aim to preserve beta-cell function
- May delay disease progression in early-diagnosed individuals
Dual and Triple Agonists
- Novel peptides targeting GLP-1/GIP/glucagon receptors
- Potential to improve glucose control and weight outcomes
Beta Cell Replacement
- Stem cell-derived islet transplantation
- Encapsulation technologies to protect grafts from immune attack
While insulin remains the cornerstone of type 1 diabetes mellitus treatment, adjunct therapies offer significant potential to optimize glycemic control, reduce insulin burden, and address comorbidities. A tailored, evidence-based approach to adjunct selection—grounded in clinical presentation, risk factors, and patient preferences—is essential. As research progresses, adjunctive options will continue to expand, paving the way for more effective and individualized care strategies for patients living with T1DM.