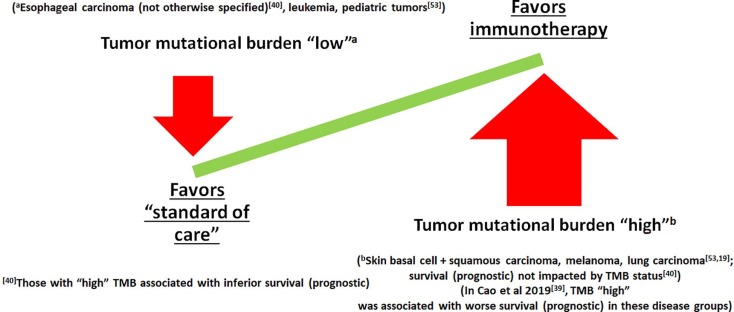
Tumor mutational burden-high (TMB-H) solid malignant tumors represent a critical biomarker category in oncology, offering actionable insights into immunotherapy response prediction and patient stratification. Defined by an elevated number of somatic mutations per megabase (mut/Mb), TMB-H has emerged as a valuable indicator for the efficacy of immune checkpoint inhibitors, particularly in treatment-refractory malignancies.
Defining Tumor Mutational Burden (TMB)
Tumor mutational burden quantifies the total number of non-synonymous somatic mutations per megabase of DNA in a tumor genome. It reflects the genomic instability of malignant cells and the potential neoantigen load available to stimulate an anti-tumor immune response.
TMB Measurement Standards
- Whole Exome Sequencing (WES): Gold standard for comprehensive TMB quantification.
- Targeted Next-Generation Sequencing (NGS) Panels: Widely adopted in clinical settings for faster, cost-effective measurement.
- High TMB Threshold: Typically ≥10 mut/Mb, though thresholds vary by tumor type and testing platform.
Mechanism of TMB in Tumor Immunogenicity
High TMB correlates with increased generation of tumor neoantigens—abnormal peptides presented by MHC molecules—which enhance recognition by cytotoxic T lymphocytes.
TMB-H tumors are more likely to overcome immune tolerance, making them responsive to immune checkpoint blockade therapy.
TMB-High Solid Malignant Tumors: A Broad Spectrum
TMB-H status is not exclusive to any single cancer type. Multiple solid tumors exhibit elevated TMB under specific genomic or environmental contexts.
Common TMB-H Tumor Types
- Non-Small Cell Lung Cancer (NSCLC): Smoking-associated mutations often increase TMB.
- Melanoma: UV radiation-induced mutagenesis elevates TMB.
- Bladder Cancer: Carcinogen exposure drives mutational load.
- Colorectal Cancer (MSI-H subset): Mismatch repair deficiency results in hypermutation.
- Head and Neck Squamous Cell Carcinoma (HNSCC): Viral and smoking-related etiologies contribute.
- Endometrial Cancer: Frequently exhibits microsatellite instability and high TMB.
Clinical Significance of TMB-H in Immunotherapy
TMB-H has been validated as a predictive biomarker for response to immune checkpoint inhibitors (ICIs), including PD-1/PD-L1 and CTLA-4 blockade.
FDA Approval
- Pembrolizumab (Keytruda): Received tumor-agnostic FDA approval for use in TMB-H solid tumors (≥10 mut/Mb) based on KEYNOTE-158 trial results.
- Approval marked a paradigm shift toward biomarker-guided immunotherapy across tumor types.
Response Rates in TMB-H Tumors
| Tumor Type | TMB-H Response Rate to ICIs |
|---|---|
| NSCLC | 45–55% |
| Melanoma | 40–50% |
| Bladder Cancer | ~25–30% |
| MSI-H Colorectal | 40–50% |
| Endometrial Cancer | ~40% |
Note: Response rates vary with other factors such as PD-L1 status and tumor microenvironment.
TMB vs. Other Biomarkers: PD-L1, MSI, and Beyond
While TMB offers valuable insights, its predictive capacity is best interpreted in combination with other biomarkers:
PD-L1 Expression
- Independent marker: TMB and PD-L1 expression can be mutually exclusive.
- Combining both improves immunotherapy stratification.
Microsatellite Instability (MSI)
- All MSI-H tumors are generally TMB-H, but not all TMB-H tumors are MSI-H.
- MSI testing complements TMB analysis for comprehensive immune profiling.
Tumor-Infiltrating Lymphocytes (TILs)
- High TMB may lead to increased TILs, but immune exclusion or suppression may still occur.
- Spatial immune contexture must be considered.
Diagnostic Strategies for Assessing TMB
Specimen Requirements
- Formalin-Fixed Paraffin-Embedded (FFPE) tissue is standard.
- Plasma-derived circulating tumor DNA (ctDNA) via liquid biopsy offers non-invasive alternative.
NGS Platforms for TMB Testing
- FoundationOne CDx
- MSK-IMPACT
- Guardant360 (liquid biopsy)
- Oncomine Tumor Mutation Load Assay
Validation of concordance between platforms is essential for accurate TMB-based decision-making.
Challenges and Limitations of TMB-H
While promising, TMB assessment and interpretation face several limitations:
1. Tumor Heterogeneity
- Intratumoral and intertumoral variation can affect TMB consistency.
2. Threshold Discrepancies
- Lack of standardization in cutoff values across platforms complicates universal application.
3. False Positives/Negatives
- Inaccurate TMB estimation may arise from low tumor purity or poor-quality DNA.
4. Non-responders with High TMB
- Not all TMB-H patients respond to ICIs due to downstream immune evasion mechanisms.
Future Directions in TMB Research
To refine TMB as a clinical tool, ongoing research focuses on:
- Pan-cancer standardization of TMB cutoffs
- Integration with immune gene expression signatures
- Multimodal biomarker models combining TMB, PD-L1, and tumor microenvironment factors
- Prospective trials evaluating TMB-guided therapeutic interventions
Tumor mutational burden-high status signifies a transformative development in the field of precision oncology. As a pan-cancer biomarker, TMB-H facilitates individualized immunotherapy approaches in diverse solid malignant tumors. Despite inherent challenges, advancements in sequencing technologies, biomarker integration, and clinical trial design continue to enhance the reliability and relevance of TMB in oncology practice.
FAQs
What defines a tumor as TMB-high?
A tumor is classified as TMB-high when it exhibits ≥10 mutations per megabase of DNA, though this threshold may vary based on cancer type and testing method.
How is TMB-high detected?
TMB is assessed using next-generation sequencing platforms on tumor tissue or circulating DNA to calculate the mutational load.
Is TMB-high predictive of response to immunotherapy?
Yes, TMB-high tumors have a greater likelihood of responding to immune checkpoint inhibitors, though outcomes may vary with other molecular factors.
Can all cancers have TMB-high status?
Potentially, though it is more common in certain types such as NSCLC, melanoma, bladder, and MSI-H colorectal cancers.
Does a high TMB guarantee treatment success?
No, while it increases the probability of response, other immune and genetic factors influence therapeutic outcomes.