Transthyretin-related amyloid cardiomyopathy (ATTR-CM) is a progressive, life-threatening form of cardiac amyloidosis caused by the deposition of misfolded transthyretin (TTR) protein in the myocardium. This condition leads to restrictive cardiomyopathy, diastolic heart failure, and ultimately, significant morbidity and mortality if left untreated.
ATTR-CM can occur in two main forms:
- Hereditary ATTR-CM (hATTR-CM): Associated with mutations in the TTR gene.
- Wild-type ATTR-CM (wtATTR-CM): Occurs without genetic mutations, primarily in elderly individuals.
Pathogenesis and Molecular Mechanism
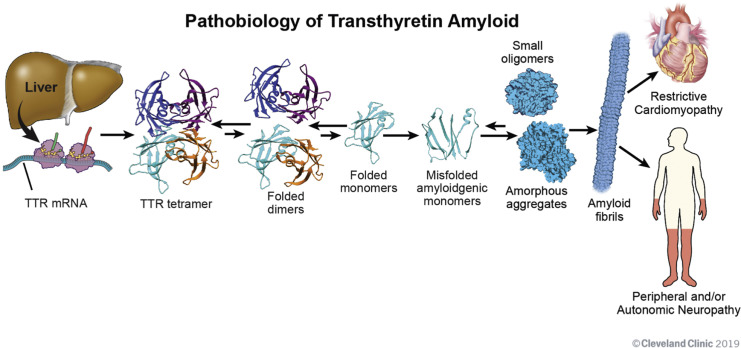
TTR is a tetrameric protein synthesized mainly by the liver. Its physiological role is to transport thyroxine (T4) and retinol-binding protein–vitamin A complex. Mutations in the TTR gene or age-related destabilization cause tetramers to dissociate into misfolded monomers, which aggregate into insoluble amyloid fibrils. These fibrils accumulate in cardiac tissues, impairing elasticity and conduction.
The continuous deposition of amyloid fibrils in the myocardium results in thickened ventricular walls, reduced compliance, and restrictive physiology—a hallmark of transthyretin amyloid cardiomyopathy.
Epidemiology and Risk Factors
- Wild-type ATTR-CM predominantly affects men over 60, often underdiagnosed and mistaken for hypertensive heart disease or hypertrophic cardiomyopathy.
- Hereditary ATTR-CM is linked to over 130 known mutations in the TTR gene; Val122Ile (V122I) is most common among individuals of African descent.
- Up to 13% of heart failure with preserved ejection fraction (HFpEF) cases may involve ATTR-CM.
Risk Factors:
- Advanced age (especially >65 years)
- Male sex
- African or Afro-Caribbean ancestry
- Family history of amyloidosis or unexplained heart failure
Clinical Manifestations of ATTR-CM
The clinical presentation is often nonspecific and may mimic other forms of cardiomyopathy, making early diagnosis challenging.
Cardiac Symptoms
- Exertional dyspnea
- Fatigue and reduced exercise tolerance
- Orthostatic hypotension
- Palpitations or syncope
- Progressive heart failure symptoms
- Arrhythmias, especially atrial fibrillation
Non-Cardiac Red Flags
- Bilateral carpal tunnel syndrome
- Spinal stenosis
- Biceps tendon rupture
- Peripheral or autonomic neuropathy (more common in hereditary forms)
Diagnostic Evaluation
1. Electrocardiogram (ECG)
- Low voltage QRS complexes despite thickened myocardium
- Pseudoinfarct patterns (Q waves in precordial leads)
2. Echocardiography
- Concentric left ventricular hypertrophy
- Small ventricular cavity
- Diastolic dysfunction with preserved EF
- Speckled or granular appearance of myocardium
3. Cardiac MRI
- Late gadolinium enhancement (LGE)
- Diffuse subendocardial or transmural uptake
- Abnormal T1 mapping and extracellular volume (ECV)
4. Bone Scintigraphy
Technetium-99m-pyrophosphate (99mTc-PYP) scintigraphy can differentiate ATTR-CM from AL amyloidosis.
- High uptake in cardiac tissue without monoclonal protein in blood/urine is diagnostic of ATTR-CM.
5. Laboratory Testing
- NT-proBNP and troponin: Elevated in most patients
- Serum and urine immunofixation + serum free light chain assay to rule out AL amyloidosis
6. Genetic Testing
- Mandatory in all ATTR-CM cases to identify hereditary mutations
- Family screening recommended for first-degree relatives
ATTR-CM vs Other Cardiomyopathies: Differential Diagnosis
| Feature | ATTR-CM | AL Amyloidosis | Hypertrophic Cardiomyopathy |
|---|---|---|---|
| Amyloid Type | Transthyretin | Light chain | None |
| Age of Onset | >60 yrs (wtATTR) | 40–60 yrs | 20–50 yrs |
| Genetic Component | Common (hATTR) | Rare | Strong |
| Organ Involvement | Heart, nerves, GI | Heart, kidney, GI | Primarily cardiac |
| Bone Scintigraphy (PYP) | Positive | Negative | Negative |
| Light Chain Abnormalities | Absent | Present | Absent |
Current Therapeutic Strategies
1. TTR Stabilizers
Tafamidis (Vyndamax/Vyndaqel)
- FDA-approved for ATTR-CM
- Binds to TTR tetramers and prevents dissociation
- Shown to reduce mortality and cardiovascular hospitalizations in the ATTR-ACT trial
Diflunisal
- Off-label NSAID that stabilizes TTR
- Not widely used due to side effects in elderly patients
2. TTR Gene Silencers
Patisiran (Onpattro)
- siRNA therapy that inhibits hepatic production of TTR
- Currently approved for hATTR polyneuropathy, under investigation for cardiac use
Inotersen (Tegsedi)
- Antisense oligonucleotide therapy reducing TTR synthesis
- Also approved for hATTR polyneuropathy
3. Investigational Therapies
- Eplontersen: Antisense therapy in phase 3 trials for ATTR-CM
- Vutrisiran: Subcutaneous siRNA with longer dosing interval
- CRISPR-Cas9 (NTLA-2001): Gene-editing therapy showing early promise
4. Supportive and Symptomatic Management
- Diuretics for fluid overload
- Beta-blockers and ACE inhibitors used with caution
- Pacemaker/ICD in cases of conduction disease
- Heart transplantation in select advanced cases
Prognosis and Survival Outlook
Without treatment, median survival for ATTR-CM is approximately 2 to 3 years from diagnosis. Tafamidis extends survival and reduces hospitalizations significantly. Early detection and initiation of therapy are critical for improved outcomes.
Genetic Counseling and Family Screening
- Essential for all patients with confirmed hereditary ATTR
- Offers risk assessment and early intervention opportunities
- Encourages cascade screening of relatives
Frequently Asked Questions:
What is the difference between wild-type and hereditary ATTR-CM?
Wild-type ATTR-CM occurs due to age-related changes in the TTR protein, while hereditary ATTR-CM is caused by specific gene mutations passed down through families.
How is ATTR-CM diagnosed?
Diagnosis involves echocardiography, cardiac MRI, nuclear imaging (PYP scan), exclusion of AL amyloidosis, and genetic testing.
Can ATTR-CM be cured?
There is no cure, but medications like Tafamidis and emerging therapies can stabilize the disease and extend survival.
Who should be tested for ATTR-CM?
Older adults with unexplained heart failure, especially with thickened ventricular walls and low ECG voltages, and individuals with carpal tunnel syndrome or family history of amyloidosis.
Is ATTR-CM hereditary?
Hereditary ATTR-CM is autosomal dominant. Family screening and genetic counseling are highly recommended if mutations are present.
Transthyretin-related amyloid cardiomyopathy is an under-recognized but increasingly diagnosable and treatable condition. Advances in imaging, genetic diagnostics, and targeted therapies like Tafamidis have reshaped its clinical outlook. Early suspicion, comprehensive evaluation, and precise treatment planning are essential to improving outcomes and preserving quality of life in patients affected by this complex disease.