TNF receptor-associated periodic fever syndrome (TRAPS) is a rare hereditary autoinflammatory disorder characterized by recurrent episodes of fever, systemic inflammation, and various other symptoms affecting the skin, eyes, muscles, and joints. It is associated with mutations in the TNFRSF1A gene, which encodes the tumor necrosis factor receptor 1 (TNFR1)—a critical component of the immune system’s inflammatory signaling pathways.
TRAPS belongs to the group of hereditary periodic fever syndromes (HPFS) and can range from mild, self-limited attacks to chronic, debilitating disease with significant organ involvement.
Genetic Basis and Pathogenesis of TRAPS
TRAPS is inherited in an autosomal dominant manner and results from mutations in the TNFRSF1A gene located on chromosome 12p13. The gene encodes TNFR1, a receptor that mediates inflammatory responses by binding to tumor necrosis factor-alpha (TNF-α).
Pathophysiology:
- Mutations cause misfolded TNFR1 proteins, leading to:
- Impaired receptor shedding
- Accumulation of intracellular TNFR1
- Constitutive activation of the inflammatory response
- These changes result in increased production of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and TNF-α, driving periodic fever and systemic symptoms.
Epidemiology and Prevalence of TRAPS
- Estimated to affect less than 1 in 1,000,000 individuals worldwide
- More commonly reported in Caucasian populations, though cases exist globally
- Onset can occur in childhood or adulthood
- No gender preference has been consistently observed
Clinical Manifestations of TRAPS
TRAPS presents with recurrent episodes of fever, typically lasting more than one week—longer than other periodic fever syndromes.
Core Symptoms:
- Recurrent high-grade fevers (lasting 5–21 days)
- Myalgia (often migratory and deep-seated)
- Abdominal pain and vomiting
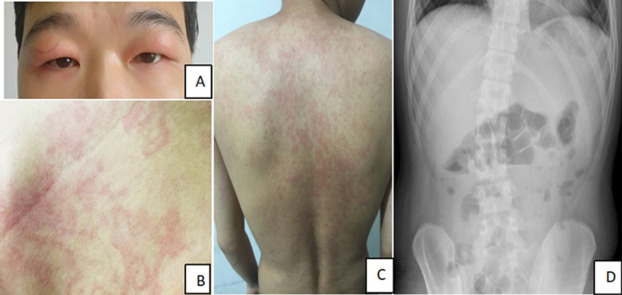
- Conjunctivitis or periorbital edema
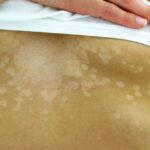
- Migratory skin rash, often erythematous and painful
- Serosal inflammation (pleuritis, peritonitis)
- Arthralgia or arthritis
- Lymphadenopathy
- Fatigue and malaise between episodes
Chronic Complications:
- Amyloidosis (AA type) is the most serious long-term complication
- Caused by persistent inflammation and elevated serum amyloid A (SAA)
- Can lead to renal failure
Diagnosis of TRAPS: Clinical and Genetic Approaches
Diagnostic Criteria:
Diagnosis is based on clinical suspicion, family history, and confirmation via genetic testing.
Laboratory Findings During Attacks:
- Elevated acute phase reactants: CRP, ESR, SAA
- Neutrophilic leukocytosis
- Absence of infection or autoimmune markers
Genetic Testing:
- Sequencing of TNFRSF1A gene confirms the diagnosis
- Specific mutations such as C30R, T50M, and R92Q are associated with variable disease severity
Differential Diagnosis:
TRAPS should be differentiated from:
- Familial Mediterranean Fever (FMF)
- Hyper IgD Syndrome (HIDS)
- Cryopyrin-Associated Periodic Syndromes (CAPS)
- Systemic juvenile idiopathic arthritis (sJIA)
- Adult-onset Still’s disease
Management and Treatment of TRAPS
There is no definitive cure for TRAPS, but targeted therapy has significantly improved patient outcomes. Treatment focuses on controlling inflammation, preventing complications, and improving quality of life.
First-Line Therapies:
1. Corticosteroids
- Effective in abortive treatment of acute attacks
- Long-term use limited by side effects and tachyphylaxis
2. IL-1 Inhibitors (Preferred for Long-Term Control)
- Anakinra (daily injection) – IL-1 receptor antagonist
- Canakinumab (monthly injection) – IL-1β monoclonal antibody
- Effective in controlling inflammation and reducing attack frequency
3. TNF Inhibitors
- Etanercept – Used with partial efficacy
- Infliximab and adalimumab – Generally avoided as they may exacerbate symptoms due to paradoxical TNF signaling
4. Colchicine
- Ineffective in TRAPS but useful for differentiation from FMF
Prognosis and Long-Term Outcomes
- Most patients lead productive lives with appropriate biologic therapy
- Untreated or poorly controlled TRAPS can result in:
- Progressive organ damage
- Renal amyloidosis
- Chronic fatigue and reduced quality of life
Monitoring:
- Regular SAA and CRP levels
- Urinalysis for proteinuria (early marker for amyloidosis)
- Genetic counseling for affected families
TNFRSF1A Mutation Variability and Clinical Correlation
Different mutations confer variable disease phenotypes:
| Mutation | Severity | Amyloidosis Risk | Notes |
|---|---|---|---|
| T50M | High | High | Early-onset, aggressive |
| R92Q | Low | Low | Often considered low-penetrance |
| C30R | Moderate | Moderate | Classical TRAPS features |
Understanding genotype-phenotype relationships aids in prognostication and treatment selection.
TRAPS in Pediatrics vs Adults
Pediatric TRAPS:
- Presents early, often within first decade
- May show more systemic symptoms
- Requires close monitoring for growth and development
Adult-Onset TRAPS:
- Often underdiagnosed or misattributed to rheumatologic conditions
- May present with more subtle, intermittent symptoms
Frequently Asked Questions:
What triggers a TRAPS flare?
Flares may be spontaneous or triggered by stress, infection, hormonal changes, or physical exertion. However, in many cases, no clear trigger is identified.
Can TRAPS be cured?
There is no known cure, but biologic therapy can control symptoms and prevent complications in the majority of cases.
Is TRAPS life-threatening?
Without treatment, TRAPS can lead to amyloidosis and organ failure, especially of the kidneys. Early diagnosis and treatment are essential.
Can TRAPS be passed to children?
Yes, TRAPS is autosomal dominant, meaning 50% chance of inheritance from an affected parent.
How often do TRAPS episodes occur?
Frequency varies from weekly to several months apart, and the duration typically ranges from 5 to 21 days.
Key Takeaways on TNF Receptor-Associated Periodic Fever Syndrome
- TRAPS is a genetic autoinflammatory disorder caused by TNFRSF1A mutations
- Characterized by long-lasting recurrent fevers, inflammation, and risk of amyloidosis
- Diagnosis requires clinical evaluation and genetic testing
- IL-1 inhibitors are the most effective long-term therapy
- Lifelong management and monitoring are essential to prevent complications