CD22-positive B-cell acute lymphoblastic leukemia (ALL) represents a subtype of leukemia characterized by the presence of the CD22 antigen on malignant B lymphoblasts. This specific marker has opened avenues for targeted therapies and advanced treatment approaches. In this article, we delve into the nature of CD22-positive ALL, covering its pathophysiology, diagnostic methods, treatment modalities, and recent advancements.
Pathophysiology of CD22-Positive ALL
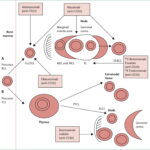
CD22 is a transmembrane glycoprotein expressed predominantly on the surface of mature B cells and their precursors. It plays a critical role in modulating B-cell receptor (BCR) signaling and maintaining immune tolerance. In CD22-positive ALL, aberrant expression of this antigen is observed on malignant B lymphoblasts. This overexpression is pivotal for the development and progression of the disease, as it contributes to the survival and proliferation of leukemic cells.
Key Features of CD22 in Leukemia:
- Cell Surface Expression: Predominantly on pre-B and mature B cells.
- Therapeutic Target: Basis for the development of monoclonal antibodies and antibody-drug conjugates.
- Diagnostic Marker: Used for flow cytometry in distinguishing B-cell lineage.
Clinical Presentation
Patients with CD22-positive ALL exhibit symptoms similar to other subtypes of ALL, including:
- Hematologic Symptoms: Anemia, thrombocytopenia, and neutropenia leading to fatigue, bruising, and infections.
- Systemic Manifestations: Fever, night sweats, and unexplained weight loss.
- Organ Involvement: Hepatosplenomegaly, lymphadenopathy, and bone pain.
Early diagnosis is critical to improving outcomes, emphasizing the importance of recognizing these symptoms promptly.
Diagnostic Approach
Flow Cytometry
Flow cytometry is a cornerstone diagnostic tool for detecting CD22 expression. A combination of immunophenotyping markers, including CD19, CD10, and CD34, helps confirm B-cell lineage.
Cytogenetic and Molecular Testing
Genetic abnormalities such as Philadelphia chromosome (t[9;22]) or rearrangements involving MLL and ETV6-RUNX1 are common in ALL. While these may not directly correlate with CD22 expression, understanding the cytogenetic landscape is crucial for risk stratification.
Bone Marrow Biopsy
A bone marrow biopsy reveals hypercellularity with a predominance of lymphoblasts, confirming the diagnosis. Immunohistochemistry further validates CD22 positivity.
Treatment Strategies
Chemotherapy
Conventional chemotherapy remains the backbone of ALL treatment, typically involving:
- Induction Phase: Combination of vincristine, corticosteroids, and anthracyclines.
- Consolidation and Maintenance: High-dose methotrexate, asparaginase, and oral maintenance therapy with 6-mercaptopurine and methotrexate.
Targeted Therapies
The emergence of CD22-targeted therapies has revolutionized treatment for CD22-positive ALL:
1. Inotuzumab Ozogamicin
An antibody-drug conjugate (ADC) linking a CD22 monoclonal antibody to calicheamicin, a potent cytotoxic agent. It binds to CD22-expressing cells, delivering the drug intracellularly and inducing apoptosis.
2. CAR-T Cell Therapy
Chimeric antigen receptor (CAR) T cells engineered to target CD22 have shown promise, particularly in relapsed/refractory cases.
Hematopoietic Stem Cell Transplantation (HSCT)
For high-risk patients or those with relapsed disease, allogeneic HSCT provides curative potential. Pre-transplant conditioning and minimal residual disease (MRD) assessment are crucial determinants of success.
Prognosis and Risk Factors
The prognosis for CD22-positive ALL depends on several factors:
- Age: Pediatric patients generally have better outcomes than adults.
- Genetic Profile: Favorable cytogenetics enhance survival rates.
- MRD Status: Achieving MRD negativity post-induction is a strong prognostic marker.
Advances in Research and Future Directions
Bispecific T-Cell Engagers (BiTEs)
Blincyto® (blinatumomab) is a BiTE that simultaneously binds CD19 on B cells and CD3 on T cells, redirecting T cells to kill malignant cells. While CD22-specific BiTEs are under investigation, their potential to complement existing therapies is immense.
Dual-Target CAR-T Cells
Efforts are underway to develop CAR-T cells targeting both CD19 and CD22 to prevent antigen escape and enhance efficacy.
Novel ADCs
Next-generation ADCs with improved linker technologies aim to increase specificity and reduce off-target toxicity.