Staphylococcal septicemia, caused predominantly by Staphylococcus aureus, is a life-threatening bloodstream infection marked by rapid progression to sepsis, organ dysfunction, and high mortality rates. The emergence of methicillin-resistant S. aureus (MRSA) strains has significantly complicated the treatment landscape. Conventional monotherapies often fail due to bacterial resistance, intracellular survival, and poor biofilm eradication. Therefore, synergistic antibiotic therapy is critical in achieving superior bactericidal outcomes and improving patient prognosis.
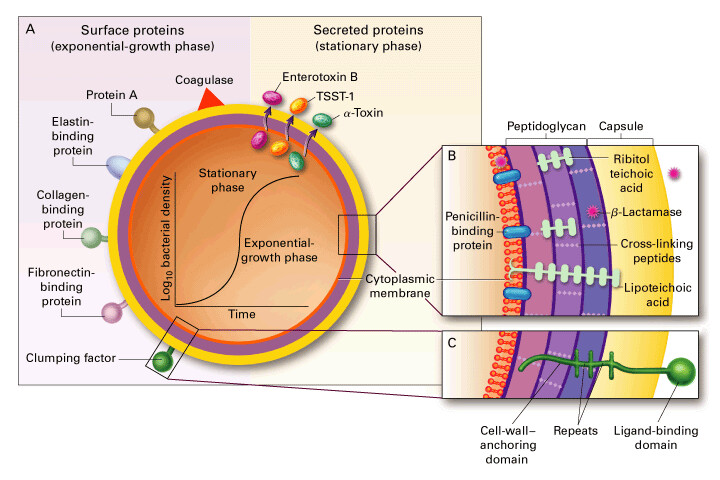
Mechanisms of Antibiotic Synergy in Staphylococcal Bloodstream Infections
Synergy between antibiotics occurs when their combined effect exceeds the sum of their individual activities. In staphylococcal septicemia, this synergy is crucial for:
- Accelerating bacterial clearance from blood
- Reducing the inoculum effect in high-burden infections
- Inhibiting biofilm formation on intravascular devices
- Preventing resistance through multi-target action
- Enhancing intracellular penetration to eliminate hidden reservoirs
Core Synergistic Antibiotic Combinations for Septicemia
Vancomycin-Based Combinations for MRSA Septicemia
Vancomycin remains a cornerstone for treating MRSA bloodstream infections. However, its limitations in bactericidal speed and tissue penetration necessitate combination therapy.
- Vancomycin + Ceftaroline
Dual inhibition of the bacterial cell wall enhances killing kinetics. This combination is effective against persistent MRSA bacteremia. - Vancomycin + Rifampin
Rifampin’s ability to penetrate biofilms and intracellular compartments complements vancomycin’s extracellular activity. Indicated especially in catheter-associated sepsis. - Vancomycin + Gentamicin
A synergistic combination that provides rapid bacterial killing. Caution is advised due to nephrotoxicity risks.
Daptomycin-Based Synergistic Strategies
Daptomycin offers potent activity against MRSA in the bloodstream, particularly when vancomycin fails. Its combinations increase efficacy in difficult cases:
- Daptomycin + Ceftaroline
A widely accepted salvage regimen for refractory MRSA septicemia. Demonstrates faster clearance and improved outcomes. - Daptomycin + Rifampin
Particularly useful in infections associated with biofilms or endovascular sources. - Daptomycin + Beta-lactams (e.g., Cefepime, Piperacillin-Tazobactam)
Enhances daptomycin’s binding to bacterial membranes, leading to superior bactericidal activity—a phenomenon known as the “see-saw effect.”
Synergistic Therapy in MSSA Septicemia
For methicillin-sensitive S. aureus (MSSA) infections, synergistic combinations can improve outcomes, particularly in high-inoculum infections or in endocarditis.
- Nafcillin + Rifampin
Proven synergy in eliminating intracellular bacteria and biofilm-associated MSSA. - Oxacillin + Daptomycin
Used in patients intolerant to first-line agents or with persistent bacteremia.
Clinical Evidence Supporting Synergistic Therapy
Multiple clinical and in vitro studies highlight the superiority of combination therapy in managing staphylococcal septicemia:
- A 2021 meta-analysis demonstrated a 25% reduction in mortality for patients treated with vancomycin + ceftaroline versus vancomycin alone in MRSA bacteremia.
- Time-kill studies reveal potent synergy between daptomycin and beta-lactams, reducing bacterial loads below detection within 8 hours.
- A retrospective cohort study showed that vancomycin-rifampin regimens significantly lowered the rate of bloodstream relapse in patients with intravascular device-associated infections.
Role of Synergy in Preventing Treatment Failure
Persistent bacteremia and septicemia relapse are significant challenges in managing S. aureus bloodstream infections. Factors contributing to treatment failure include:
- Delayed source control
- Biofilm-mediated immune evasion
- Suboptimal monotherapy in high bacterial load
- Inadequate tissue penetration by single agents
Synergistic therapy addresses these issues by amplifying drug activity at the infection site and reducing bacterial persistence in protected niches such as heart valves, joints, or central nervous system tissue.
Monitoring and Duration of Synergistic Treatment
Appropriate antimicrobial stewardship and close monitoring are essential during combination therapy to mitigate toxicity risks and ensure efficacy.
- Blood Cultures: Should be drawn every 24–48 hours until negative.
- Inflammatory Markers: C-reactive protein (CRP) and procalcitonin help assess therapeutic response.
- Renal and Hepatic Function: Must be closely tracked during gentamicin or rifampin co-administration.
- Duration: Treatment typically extends 14–28 days, depending on the source of infection, complications, and response to therapy.
Personalized Medicine and the Future of Synergistic Septicemia Treatment
The field is evolving toward precision-guided therapies supported by rapid diagnostics and synergy testing. Advanced approaches include:
- Rapid Molecular Diagnostics: Identifies resistance patterns to tailor combinations.
- E-test and Checkerboard Assays: Clinically relevant synergy tests to optimize pairing.
- Pharmacokinetic/Pharmacodynamic (PK/PD) Modeling: Ensures optimal dosing of combinations.
- Adjunctive Therapies: Bacteriophage-antibiotic synergy and monoclonal antibodies under clinical investigation.
Staphylococcal septicemia demands urgent, aggressive, and targeted antimicrobial management. Synergistic antibiotic therapy has emerged as an essential strategy to improve bacterial clearance, reduce complications, and prevent relapse. Whether dealing with MRSA, MSSA, or device-associated infections, the application of evidence-based combination regimens is vital to enhancing treatment success. Future strategies integrating rapid diagnostics, synergy testing, and host-specific factors promise even more effective outcomes in bloodstream infection control.