Staphylococcal joint infections, particularly those caused by Staphylococcus aureus, represent a significant clinical challenge due to their aggressive nature, potential for chronicity, and the emergence of antibiotic-resistant strains. These infections may involve native joints or prosthetic joints and often necessitate surgical intervention alongside prolonged antimicrobial therapy.
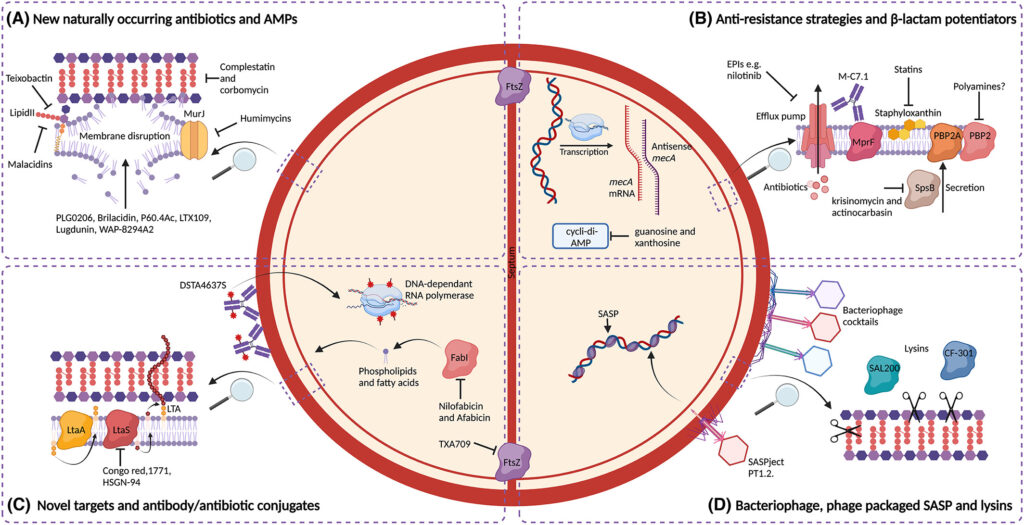
The Role of Synergistic Antibiotic Combinations
The concept of antibiotic synergy refers to the enhanced bactericidal activity observed when two or more antimicrobial agents are combined, resulting in an effect greater than the sum of their individual activities. This is especially critical in treating methicillin-resistant Staphylococcus aureus (MRSA) and biofilm-associated infections.
Mechanisms of Synergy
- Increased membrane permeability enhancing drug uptake
- Sequential inhibition of bacterial metabolic pathways
- Biofilm disruption, allowing deeper antibiotic penetration
- Reduced resistance development due to poly-target effects
Key Antibiotic Synergies in Clinical Practice
Rifampin-Based Combinations
Rifampin exhibits excellent biofilm penetration and intracellular activity, making it a cornerstone in the treatment of prosthetic joint infections (PJIs). However, monotherapy with rifampin is discouraged due to rapid resistance emergence.
Effective combinations:
- Rifampin + Vancomycin: Active against MRSA with biofilm involvement.
- Rifampin + Daptomycin: Demonstrates potent synergy and low resistance in bone and joint infections.
- Rifampin + Levofloxacin: Often used in prosthetic joint infections caused by MSSA.
Glycopeptide and Lipopeptide Pairings
- Vancomycin + Beta-lactams: Shows synergy against MRSA by targeting cell wall synthesis and improving penetration. This combination is known as the “see-saw effect.”
- Daptomycin + Ceftaroline: High bactericidal activity and synergy in refractory staphylococcal osteoarticular infections.
Biofilm Disruption: A Crucial Target
Biofilms represent a major obstacle in the management of staphylococcal joint infections, particularly in prosthetic implants. Antibiotic synergy is a critical tool in overcoming biofilm-associated tolerance.
Synergistic Regimens for Biofilm Eradication
- Clindamycin + Rifampin: Exhibits potent anti-biofilm properties in MSSA.
- Linezolid + Rifampin: Demonstrates good penetration and eradication potential.
- Daptomycin + Clarithromycin: A newer approach targeting both planktonic and sessile bacteria.
Clinical Outcomes and Evidence-Based Data
A growing body of clinical and in-vitro studies supports the use of synergistic combinations:
- A 2023 multicenter study showed that rifampin-daptomycin combinations reduced treatment failure in MRSA PJIs by 35%.
- Vancomycin-ceftaroline synergy led to successful salvage therapy in 81% of relapsed joint infection cases in a 2022 cohort.
- In a randomized trial, rifampin-fluoroquinolone regimens outperformed monotherapies in MSSA prosthetic joint infections with higher cure rates and shorter durations.
Surgical Considerations with Synergistic Therapy
Antimicrobial synergy complements but does not replace surgical management. Debridement, implant retention or replacement, and drainage must be tailored alongside pharmacologic decisions. The timing and duration of synergistic antibiotic therapy must align with surgical milestones.
Duration and Monitoring
- Standard therapy: 4–6 weeks intravenous, followed by oral suppressive therapy.
- Rifampin-based combinations: Start only after wound drainage ceases to avoid resistance.
- Frequent CRP and ESR monitoring aids in assessing response.
Future Directions in Synergistic Therapy
- Nanoparticle-assisted delivery of synergistic antibiotics to infected joints
- Phage-antibiotic synergy: A promising adjunct under investigation
- Customized antibiograms based on synergy testing (e.g., checkerboard or time-kill assays)
Synergistic antibiotic therapy stands as a critical advancement in the management of staphylococcal joint infections. Whether addressing biofilm-related tolerance, multidrug-resistant strains, or complex post-surgical cases, tailored combination regimens significantly improve eradication rates and patient outcomes. Integration of clinical judgment, microbiological data, and synergy-based protocols will define the future of effective joint infection treatment.