Staphylococcal infections, primarily caused by Staphylococcus aureus and coagulase-negative staphylococci (CoNS), represent a significant global burden due to their capacity for virulence, biofilm formation, and antibiotic resistance. Methicillin-resistant S. aureus (MRSA) further complicates treatment, necessitating combination regimens that provide synergistic effects. These strategies are critical for severe infections, including bacteremia, endocarditis, osteomyelitis, and device-associated infections.
The phenomenon of synergy—where two or more antibiotics work together to achieve superior bactericidal effects—has become essential in combating resistant staphylococcal strains, minimizing treatment failure, and suppressing resistance development.
Mechanisms of Synergy in Staphylococcal Infections
Synergistic effects result from the combination of agents that disrupt multiple bacterial targets simultaneously. This multipronged approach weakens bacterial defenses and enhances antibiotic penetration, particularly in the context of biofilms and intracellular survival.
Core Mechanisms:
- Cell Wall Disruption: Beta-lactams and glycopeptides inhibit peptidoglycan synthesis, destabilizing the bacterial cell wall.
- Membrane Depolarization: Lipopeptides like daptomycin disrupt membrane potential.
- Protein Synthesis Inhibition: Aminoglycosides and oxazolidinones interfere with ribosomal activity.
- RNA Synthesis Inhibition: Rifampin blocks transcription and penetrates biofilms effectively.
Established Synergistic Combinations for Staphylococcal Infections
1. Vancomycin + Gentamicin
- Indication: MRSA endocarditis and deep-seated infections
- Synergistic Effect: Vancomycin weakens cell wall, facilitating gentamicin entry for enhanced bactericidal action
- Limitation: Ototoxicity and nephrotoxicity risk; duration restricted to ≤5 days
2. Daptomycin + Ceftaroline
- Indication: Persistent MRSA bacteremia, salvage therapy
- Mechanism: Ceftaroline restores daptomycin’s binding in daptomycin non-susceptible strains
- Evidence: Strong clinical and in vitro synergy; lower daptomycin MICs
3. Nafcillin + Gentamicin
- Indication: MSSA bacteremia, native valve endocarditis
- Effect: Rapid bacteremia clearance and lower relapse rate
- Use: Limited to 3–5 days due to aminoglycoside toxicity
4. Rifampin + Linezolid
- Indication: Biofilm-associated infections, prosthetic devices
- Effect: Linezolid targets intracellular bacteria, rifampin penetrates biofilms
- Note: Avoid rifampin monotherapy to prevent rapid resistance
5. Fosfomycin + Daptomycin
- Indication: MRSA with biofilm involvement
- Effect: Fosfomycin enhances daptomycin’s bactericidal effect
- Advantage: Effective against daptomycin-nonresponsive strains
Synergy in the Treatment of Biofilm-Associated Staphylococcal Infections
Biofilms act as a barrier against host defenses and antibiotics, making monotherapy often insufficient. Synergistic regimens, particularly those incorporating rifampin, are essential in these contexts.
Strategies:
- Vancomycin or Daptomycin + Rifampin: Enhanced activity against sessile bacteria
- Linezolid + Rifampin: Effective for intracellular and prosthesis-related infections
- Clindamycin + Rifampin: Used in orthopedic and vascular graft infections
Synergistic Therapy in Multidrug-Resistant Staphylococcus aureus
Resistance mechanisms in MRSA—including altered penicillin-binding proteins (PBP2a), biofilm formation, and small colony variants—demand innovative synergistic therapies.
Promising Combinations:
- Daptomycin + Ceftaroline: Emerging salvage therapy standard for persistent MRSA
- Linezolid + Daptomycin: Targets both protein synthesis and membrane integrity
- Oritavancin + Rifampin: Long-acting agents under investigation for synergy
In Vitro and Clinical Evidence Supporting Synergy
Multiple studies have validated the clinical benefits of synergistic antibiotic combinations:
- Time-Kill Studies: Demonstrate ≥2-log reduction in CFU with combinations
- Animal Models: Show increased survival and bacterial clearance
- Clinical Meta-Analyses: Report reduced mortality and bacteremia duration with combinations like daptomycin-ceftaroline
Synergy testing methods include checkerboard assays, fractional inhibitory concentration (FIC) indices, and time-kill curves.
Monitoring and Adverse Effects in Combination Therapy
While synergy offers potent therapeutic benefits, it must be balanced against potential toxicities and pharmacologic interactions.
Considerations:
- Gentamicin: Nephrotoxicity and ototoxicity; monitor serum levels and renal function
- Rifampin: Hepatotoxicity and CYP450 interactions
- Linezolid: Risk of thrombocytopenia and serotonin syndrome
- Daptomycin: Monitor creatine kinase levels for muscle toxicity
Close therapeutic drug monitoring (TDM) and pharmacokinetic optimization are critical during prolonged combination regimens.
Personalized Synergistic Therapy: The Future of Staphylococcal Infection Management
Emerging technologies enable more precise tailoring of synergistic treatments:
- Rapid Diagnostics: Real-time detection of resistance genes and biofilm presence
- AI-Driven Synergy Prediction Models: Machine learning tools to identify optimal drug pairings
- Pharmacodynamic Modeling: Simulates in vivo responses for optimized regimens
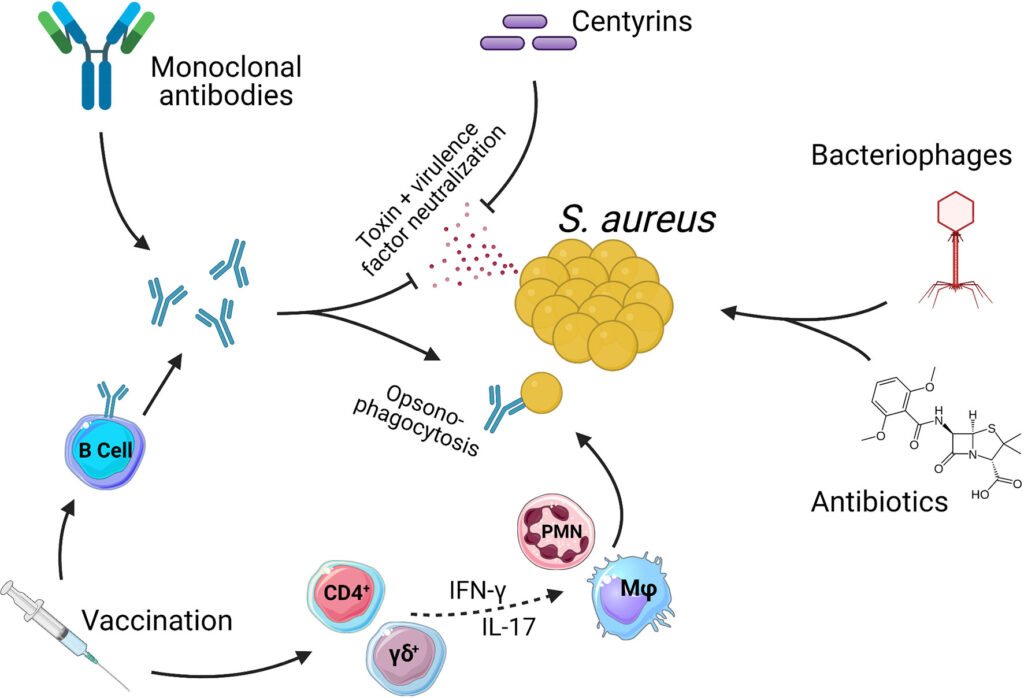
- Adjunctive Therapies: Bacteriophage-antibiotic combinations and monoclonal antibodies in development
Synergistic antibiotic therapy is a cornerstone in the modern management of staphylococcal infections, especially in resistant or device-associated cases. By combining agents that target complementary bacterial mechanisms, we achieve superior eradication, prevent resistance development, and improve clinical outcomes. Clinical decisions should be guided by infection site, pathogen profile, and individual patient risk factors.