Pseudomonas aeruginosa is a Gram-negative, opportunistic pathogen notorious for its role in chronic and hard-to-treat infections, especially osteomyelitis. Its ability to form biofilms, resist multiple antibiotics, and adapt to the bone microenvironment makes it a formidable organism in orthopedic and post-traumatic infections. Osteomyelitis caused by P. aeruginosa often necessitates prolonged antimicrobial therapy and, in some cases, surgical debridement.
Challenges in Treating P. aeruginosa Bone Infections
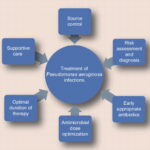
Effective treatment of P. aeruginosa osteomyelitis is impeded by:
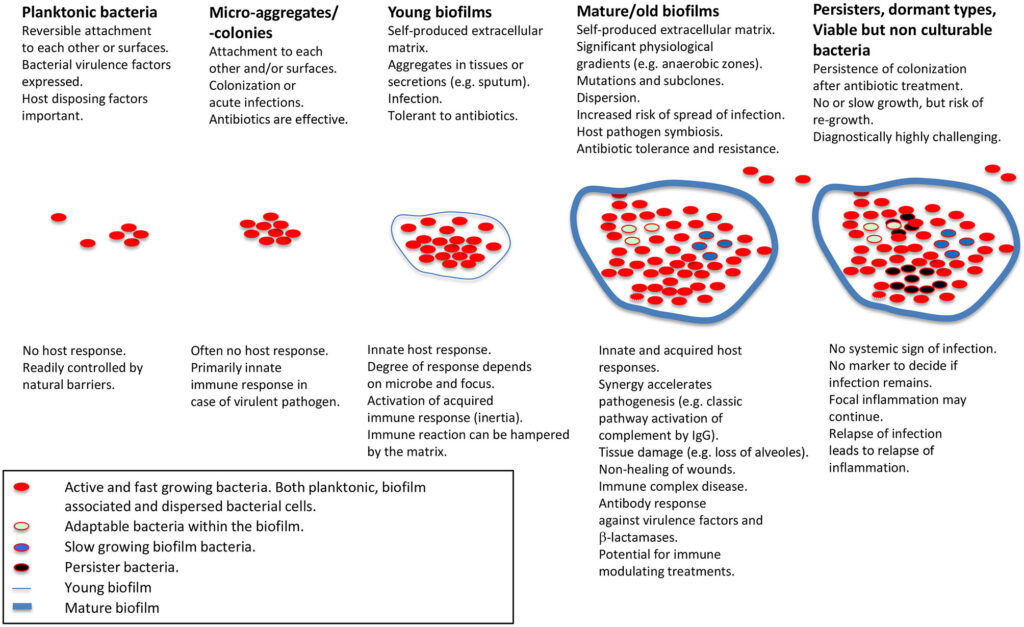
- Biofilm formation: Inhibits immune response and reduces antibiotic penetration.
- Multidrug resistance (MDR): Limits monotherapeutic options.
- Poor bone penetration of antibiotics: Compromises efficacy at infection sites.
- Intracellular survival mechanisms: Leads to persistent infection despite systemic treatment.
Antibiotic Synergy: A Critical Strategy Against Resistance
Antibiotic synergy refers to the combined effect of two or more antibiotics producing greater antimicrobial activity than the sum of their individual effects. This approach is particularly beneficial in overcoming P. aeruginosa‘s robust defense mechanisms and achieving eradication in osteomyelitic bone tissue.
Mechanisms of Synergistic Antibiotic Interactions
- Cell wall disruption + enhanced intracellular entry
- Inhibition of multiple bacterial targets
- Efflux pump inhibition
- Biofilm matrix penetration improvement
Synergistic Combinations for Pseudomonas aeruginosa Osteomyelitis
β-Lactam and Aminoglycoside Combinations
Meropenem + Amikacin
This combination demonstrates potent synergy, especially in isolates resistant to β-lactams alone. Meropenem disrupts the cell wall, facilitating increased uptake of amikacin, which inhibits protein synthesis.
Piperacillin-Tazobactam + Tobramycin
Exhibits enhanced bactericidal activity and is frequently used in severe osteomyelitis cases where surgical access is limited.
Fluoroquinolone and β-Lactam Synergy
Ciprofloxacin + Ceftazidime
This combination improves intracellular killing. Ciprofloxacin provides intracellular coverage, while ceftazidime disrupts cell wall synthesis.
Colistin-Based Synergy for MDR Isolates
Colistin + Rifampicin
Colistin disrupts the bacterial outer membrane, increasing rifampicin’s penetration. This combination has shown in-vitro synergy against extensively drug-resistant (XDR) P. aeruginosa, including strains within biofilms.
Fosfomycin as a Synergistic Agent
Fosfomycin has emerged as a potent synergistic partner due to its ability to inhibit early stages of cell wall synthesis and enhance penetration of co-administered agents.
In-Vitro Evidence Supporting Antibiotic Synergy
Studies utilizing time-kill assays, checkerboard analysis, and E-test strip methodologies consistently demonstrate:
- ≥2 log₁₀ CFU/mL reductions when antibiotics are combined
- Significant improvements in Minimum Inhibitory Concentrations (MICs)
- Enhanced eradication of biofilm-embedded bacteria
Clinical Application of Synergistic Therapy in Osteomyelitis
Case Management Considerations
- Empirical therapy: Should consider local resistance patterns and start with a synergistic regimen.
- Definitive therapy: Guided by culture sensitivity, aiming for combinations that ensure deep bone penetration and activity against biofilm forms.
- Surgical debridement: Often necessary to enhance antibiotic efficacy.
Duration of Therapy
Combination regimens are typically administered for 6 to 12 weeks, with IV therapy followed by oral synergistic agents, especially fluoroquinolones.
Emerging Synergy Models and Experimental Approaches
Nanoparticle-Aided Delivery Systems
Combining nanoparticles with antibiotics offers targeted delivery to bone tissues, increasing local drug concentration and reducing systemic toxicity.
Phage-Antibiotic Synergy (PAS)
Bacteriophage therapy in conjunction with antibiotics shows promising results in preclinical models for eradicating P. aeruginosa biofilms in osteomyelitis.
Antimicrobial Peptides + Antibiotics
Experimental studies demonstrate that peptides such as LL-37 enhance the uptake and bactericidal effect of conventional antibiotics.
The use of synergistic antibiotic therapy for Pseudomonas aeruginosa osteomyelitis represents a cornerstone in overcoming resistance and achieving successful clinical outcomes. Careful selection of antibiotic pairs based on pharmacokinetics, bone penetration, and local susceptibility patterns is essential. Future advances, including targeted delivery and phage therapy, are poised to redefine synergy in chronic bone infection management.