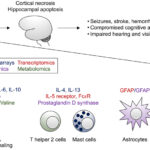
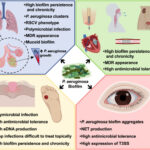
Nosocomial pneumonia, particularly ventilator-associated pneumonia (VAP), remains one of the most prevalent and severe hospital-acquired infections. Pseudomonas aeruginosa, a non-fermenting Gram-negative bacillus, ranks among the top etiologic agents, especially in intensive care units (ICUs). Its remarkable ability to develop multidrug resistance (MDR) and form biofilms makes treatment highly challenging. Hence, synergistic antibiotic therapy has emerged as a crucial strategy to enhance clinical efficacy, suppress resistance, and reduce mortality.
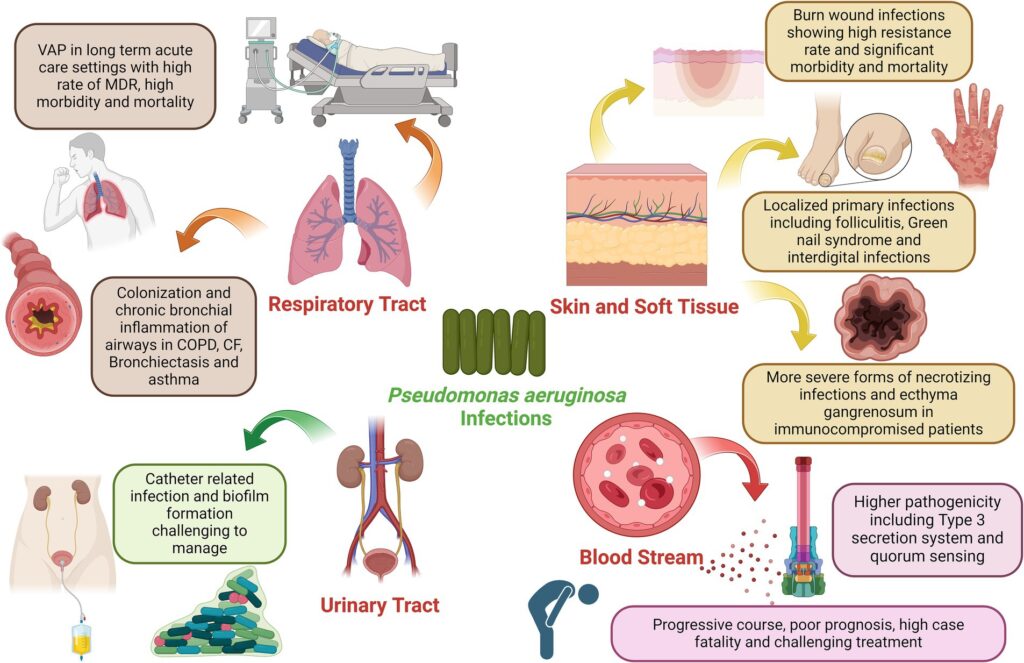
Pathophysiology and Resistance Mechanisms of Pseudomonas aeruginosa
The intrinsic resistance of P. aeruginosa is attributed to its low outer membrane permeability, efflux pumps (notably MexAB-OprM), and inducible β-lactamases. Acquired resistance further complicates therapy through the production of metallo-β-lactamases, porin mutations (e.g., OprD loss), and horizontal gene transfer. These features collectively lead to resistance against carbapenems, aminoglycosides, and fluoroquinolones—often necessitating combination regimens.
Mechanisms and Rationale for Antibiotic Synergy
Synergy occurs when the combined effect of two or more antibiotics is significantly greater than the sum of their individual activities. This phenomenon can:
- Enhance bacterial killing
- Lower individual drug dosages
- Delay or prevent emergence of resistance
- Improve clinical outcomes in critically ill patients
Key mechanisms include:
- Sequential inhibition of bacterial pathways (e.g., β-lactams with aminoglycosides)
- Membrane permeabilization, allowing increased intracellular concentration (e.g., colistin with rifampin)
- Biofilm disruption, enabling better penetration and efficacy
Evidence-Based Synergistic Combinations Against P. aeruginosa
β-Lactam and Aminoglycoside Synergy
This combination exploits the β-lactam’s cell wall disruption to facilitate aminoglycoside entry. Clinical studies demonstrate improved bactericidal activity and reduced relapse rates. Common combinations include:
- Piperacillin-tazobactam + Amikacin
- Ceftazidime + Tobramycin
These regimens show enhanced efficacy particularly in non-carbapenemase-producing strains.
β-Lactam and Fluoroquinolone Combinations
Although less frequently used due to fluoroquinolone resistance, synergy is observed when both agents are active. Examples:
- Cefepime + Levofloxacin
- Meropenem + Ciprofloxacin
These may be reserved for strains with intermediate susceptibility or used in de-escalation protocols.
Colistin-Based Combinations
Colistin remains a last-resort agent against MDR-P. aeruginosa. Its synergy with other antibiotics is vital for achieving bactericidal effects:
- Colistin + Meropenem
- Colistin + Rifampin
- Colistin + Fosfomycin
These combinations are especially effective against extensively drug-resistant (XDR) isolates, though nephrotoxicity must be monitored closely.
Novel and Investigational Synergistic Approaches
β-Lactam/β-Lactamase Inhibitor Combinations
Emerging agents like ceftolozane-tazobactam and ceftazidime-avibactam have redefined treatment landscapes by overcoming resistance mediated by ESBLs and AmpC β-lactamases.
- Ceftolozane-tazobactam + Amikacin shows potent in vitro and in vivo synergy.
- Ceftazidime-avibactam + Aztreonam is under investigation for dual β-lactam synergy in MBL-producing strains.
Phage and Antibiotic Combinations
Phage therapy combined with antibiotics demonstrates promising synergy in reducing biofilm-embedded P. aeruginosa populations. Though largely experimental, early-phase trials indicate potential for adjunctive use.
Clinical Implementation and Stewardship Considerations
Optimal application of synergy in clinical settings demands:
- Antimicrobial susceptibility testing (AST) including time-kill and checkerboard assays
- Pharmacodynamic modeling to determine time above MIC, peak/MIC ratios
- Therapeutic drug monitoring (TDM) for agents like aminoglycosides and colistin
- Infection control policies to prevent transmission of MDR strains
Antimicrobial stewardship must guide judicious use, tailoring combinations based on resistance profiles and site-specific pharmacokinetics.
Outcomes of Synergistic Therapy in Nosocomial Pneumonia
Multiple studies validate the efficacy of combination therapy in reducing mortality, shortening ICU stays, and improving microbiological eradication. Meta-analyses suggest a mortality benefit in severe pneumonia when synergy is appropriately employed, particularly in septic shock or immunocompromised populations.
Optimizing Synergy to Combat P. aeruginosa Pneumonia
Given the complex resistance mechanisms of Pseudomonas aeruginosa, synergy-driven combination therapy represents a cornerstone of effective management in nosocomial pneumonia. Emerging agents and intelligent stewardship frameworks further enhance our ability to combat this formidable pathogen. Clinical success hinges on timely initiation, targeted combinations, and individualized pharmacotherapy, ensuring optimal patient outcomes.