Sulfadiazine, a sulfonamide antimicrobial agent, is widely used in the treatment of various bacterial infections and toxoplasmosis, often in combination with pyrimethamine. Despite its therapeutic efficacy, sulfadiazine has a well-documented risk of toxicity, especially in patients with predisposing conditions. Sulfadiazine toxicity encompasses a broad range of adverse effects, ranging from mild hypersensitivity reactions to life-threatening organ dysfunction. Understanding the clinical implications, risk factors, and strategies for prompt intervention is crucial to ensuring patient safety.
Overview of Sulfadiazine and Its Clinical Use
Sulfadiazine belongs to the sulfonamide class of antibiotics, exerting its antimicrobial effect by inhibiting dihydropteroate synthase, a key enzyme in folate synthesis in bacteria. It is particularly effective in the management of:
- Toxoplasmosis (especially cerebral toxoplasmosis in immunocompromised patients)
- Urinary tract infections
- Rheumatic fever prophylaxis
- Burn wound infections (topical silver sulfadiazine)
While generally safe when used within therapeutic doses, sulfadiazine carries a risk of toxicity that can be exacerbated by prolonged use, high doses, or impaired drug clearance.
Mechanism of Sulfadiazine Toxicity
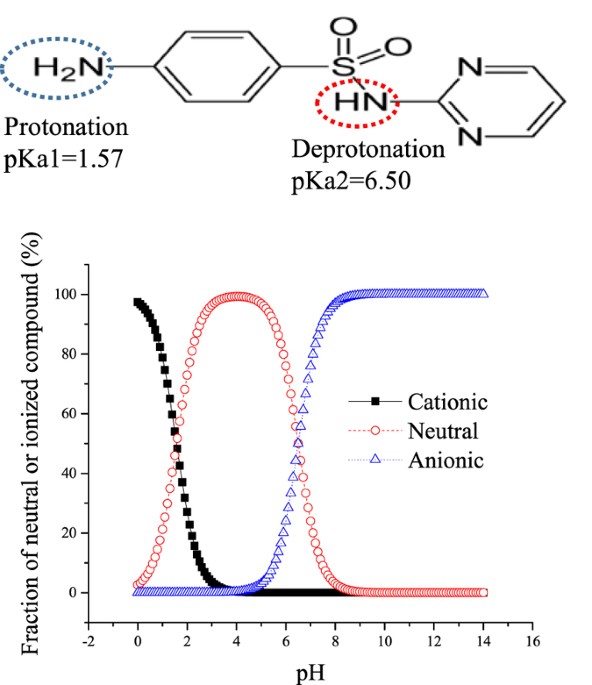
Toxicity results from the drug’s metabolism and excretion pathways. Sulfadiazine is primarily eliminated via the kidneys. Its poor solubility in acidic urine can lead to crystal precipitation in renal tubules, resulting in obstructive nephropathy. Additionally, its metabolism into reactive intermediates can cause immune-mediated responses and direct tissue injury.
Clinical Manifestations of Sulfadiazine Toxicity
1. Renal Toxicity and Crystalluria
The most common serious complication of sulfadiazine is renal toxicity due to crystal-induced nephropathy.
- Symptoms: Flank pain, hematuria, decreased urine output, renal colic
- Laboratory Findings: Elevated serum creatinine and BUN, urinalysis showing crystals
- Pathophysiology: Formation of poorly soluble acetylated metabolites in acidic urine
2. Hypersensitivity Reactions
Sulfadiazine is known to provoke severe immune responses, including:
- Stevens-Johnson Syndrome (SJS)
- Toxic Epidermal Necrolysis (TEN)
- Drug Rash with Eosinophilia and Systemic Symptoms (DRESS)
Patients may initially present with fever, rash, malaise, and lymphadenopathy.
3. Hematologic Complications
Long-term use can result in bone marrow suppression, especially in individuals with folate deficiency.
- Agranulocytosis
- Aplastic anemia
- Hemolytic anemia (notably in patients with G6PD deficiency)
4. Hepatotoxicity
Sulfadiazine-induced liver injury may manifest as:
- Cholestatic hepatitis
- Elevated liver enzymes
- Jaundice
Though rare, hepatotoxicity is potentially severe and may necessitate discontinuation.
5. Neurological Symptoms
CNS effects are uncommon but may include:
- Headache
- Dizziness
- Ataxia
- Seizures (in high-dose regimens, especially with pyrimethamine)
Risk Factors for Sulfadiazine Toxicity
Identifying patients at increased risk is essential for prevention and early intervention.
- Dehydration and acidic urine
- Renal impairment
- Folate deficiency
- Genetic predisposition (e.g., slow acetylators)
- Concomitant use of nephrotoxic or hepatotoxic drugs
- Immunocompromised status (e.g., HIV/AIDS)
Diagnostic Evaluation
Early detection of sulfadiazine toxicity relies on vigilant monitoring and targeted investigations.
Clinical Assessment
- Detailed drug history and symptom review
- Physical examination (rash, mucosal lesions, hepatosplenomegaly)
Laboratory Investigations
- Renal Function Tests: BUN, serum creatinine, urinalysis
- Complete Blood Count: Monitor for anemia, leukopenia, thrombocytopenia
- Liver Function Tests: AST, ALT, ALP, bilirubin
- Electrolytes and acid-base status
Urinalysis and Microscopy
Detection of sulfadiazine crystals—needle-shaped or sheaf-like, yellow-brown in color—is pathognomonic in cases of crystalluria.
Management of Sulfadiazine Toxicity
Immediate Discontinuation
The cornerstone of management is prompt withdrawal of sulfadiazine at the earliest sign of toxicity.
Supportive Treatment
- Hydration: Forced alkaline diuresis with IV fluids and sodium bicarbonate to prevent crystal formation
- Electrolyte Correction
- Pain control and antipyretics
Specific Interventions
- Hemodialysis: May be required in severe renal impairment or for rapid drug clearance
- Corticosteroids: For severe hypersensitivity reactions (SJS, TEN)
- Folate Supplementation: Especially when co-administered with pyrimethamine
Alternative Therapy
In cases where sulfadiazine must be discontinued, substitute antimicrobials may include:
- Clindamycin (in toxoplasmosis)
- Trimethoprim-sulfamethoxazole (cautiously, due to similar structure)
- Atovaquone or azithromycin
Prevention Strategies
- Maintain high fluid intake during therapy
- Alkalinize urine using potassium citrate or bicarbonate
- Routine laboratory monitoring for high-risk patients
- Avoid use in patients with known sulfa allergy
- Educate patients about early signs of adverse effects
Prognosis and Long-Term Outcomes
Most patients recover fully upon cessation of the drug and appropriate treatment. However, delayed recognition of toxicity may result in irreversible organ damage or death, particularly in cases of TEN or fulminant hepatic failure.
Sulfadiazine toxicity, while preventable, remains a serious complication of sulfonamide therapy. Clinicians must exercise caution when prescribing this agent, especially in high-risk populations. Early identification, appropriate laboratory monitoring, and timely intervention are vital to mitigate life-threatening outcomes. With comprehensive patient education and clinical vigilance, the therapeutic benefits of sulfadiazine can be maximized while minimizing its toxic potential.