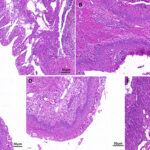
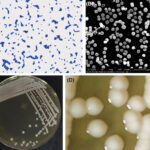
Staphylococcus epidermidis, a coagulase-negative staphylococcus (CoNS), is part of the normal human skin microbiota. While generally non-pathogenic in healthy individuals, it has emerged as a significant opportunistic pathogen in skin and skin structure infections (SSSIs), especially in immunocompromised patients and those with indwelling medical devices. Its ability to form biofilms and resist antibiotics presents a clinical challenge in diagnosis and treatment.
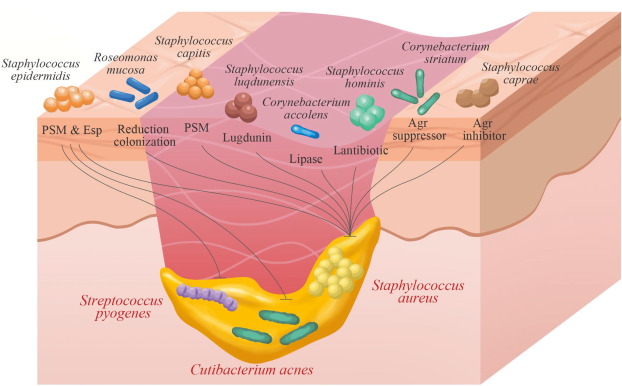
Microbiology and Virulence Mechanisms
S. epidermidis is a gram-positive, facultative anaerobe that resides on skin and mucosal surfaces. Unlike S. aureus, it lacks many aggressive virulence factors but compensates through:
- Biofilm formation: Facilitates adhesion to surfaces and resistance to host immunity.
- Slime production: Aids in persistence on prosthetics and catheters.
- Antibiotic resistance genes: Notably mecA, conferring methicillin resistance (MRSE).
These features allow S. epidermidis to colonize wounds and surgical sites, particularly in hospitalized or immunocompromised patients.
Risk Factors for Skin and Skin Structure Infections by S. epidermidis
The likelihood of developing S. epidermidis-related skin infections increases under the following conditions:
- Presence of prosthetic devices or catheters
- Recent surgical incisions or trauma
- Compromised immune systems (e.g., diabetes, cancer, HIV)
- Prolonged hospital stays
- Skin barrier disruption (eczema, burns, dermatitis)
Clinical Presentations of S. epidermidis Skin Infections
Common Manifestations:
- Cellulitis: Localized redness, swelling, pain, and warmth
- Surgical site infections: Postoperative inflammation, delayed healing
- Wound infections: Discharge, odor, tissue breakdown
- Device-related infections: Erythema, tenderness at device site, systemic symptoms
- Chronic ulcer colonization: Diabetic foot ulcers or pressure sores
These infections are often insidious and less aggressive than those caused by S. aureus, making clinical suspicion and microbiological confirmation essential.
Diagnostic Evaluation
Clinical Assessment
- Visual inspection for signs of inflammation, discharge, or necrosis
- Palpation for induration or fluctuance suggesting abscess formation
Laboratory Workup
- Wound culture: Essential for confirming pathogen identity and antibiotic susceptibility
- Gram stain: May reveal gram-positive cocci in clusters
- Blood cultures: Indicated in systemic signs or device-related infections
- CBC and CRP/ESR: Nonspecific but useful for gauging systemic involvement
Imaging
- Ultrasound or MRI: To evaluate for deeper involvement such as abscesses or osteomyelitis in complex or chronic infections
Biofilm Formation in Device-Associated Infections
Biofilm formation remains the hallmark of chronic S. epidermidis infections, particularly in surgical implants, catheters, and chronic ulcers. It renders standard antibiotic therapy less effective and often necessitates device removal or surgical debridement.
Antimicrobial Therapy
Empiric Treatment
Initial empiric therapy should cover methicillin-resistant CoNS, especially in healthcare-associated infections.
Common Options:
- Vancomycin (IV): First-line for suspected MRSE
- Linezolid (oral/IV): Effective in both community and hospital settings
- Daptomycin (IV): Alternative for vancomycin-intolerant patients
- Clindamycin or doxycycline: For mild to moderate infections pending susceptibility
Targeted Therapy
Following culture results, narrow therapy to effective agents. For methicillin-sensitive strains (MSSE), nafcillin, oxacillin, or cefazolin are preferred. Duration typically ranges from 7–14 days, adjusted for severity and response.
Surgical and Supportive Interventions
- Wound debridement: Removal of necrotic tissue and biofilm-encased bacteria
- Drainage of abscesses: Essential for resolution in purulent infections
- Removal of infected devices: In recalcitrant or relapsing infections
- Wound care and dressings: Maintain moist environment and barrier protection
- Adjunct therapies: Negative pressure wound therapy (NPWT), topical antiseptics
Resistance Patterns and Infection Control
S. epidermidis exhibits increasing resistance to beta-lactams and fluoroquinolones. Infection control measures are vital:
- Hand hygiene
- Aseptic techniques during catheter insertion and dressing changes
- Decolonization protocols in high-risk units
- Surveillance cultures in outbreak scenarios
Complications of Chronic or Invasive Infections
- Chronic non-healing ulcers
- Sepsis in immunocompromised patients
- Endovascular infection from hematogenous spread
- Prosthetic joint infection (PJI)
- Recurrent abscesses
Persistent infection may necessitate multidisciplinary management, including infectious disease consultation, surgical teams, and wound care specialists.
Prevention Strategies for Healthcare and Community Settings
- Use of chlorhexidine for preoperative skin preparation
- Early detection and replacement of compromised devices
- Timely removal of unnecessary catheters or lines
- Patient education on wound hygiene and monitoring signs of infection
- Antibiotic stewardship to reduce resistance emergence
Prognosis
With timely diagnosis and proper intervention, the prognosis for most S. epidermidis skin and soft tissue infections is favorable. Delays in treatment or inappropriate therapy increase the risk of complications, particularly in immunocompromised individuals or patients with foreign body implants.
Staphylococcus epidermidis, although part of normal flora, represents a significant cause of skin and skin structure infections in vulnerable populations. Its capacity for biofilm formation, antibiotic resistance, and chronic persistence demands a strategic, evidence-based approach to diagnosis and therapy. Optimizing clinical outcomes requires a combination of accurate microbiological identification, targeted antibiotic therapy, and in many cases, surgical or procedural intervention.