Solid tumors with NTRK gene fusion represent a rare but actionable subset of malignancies driven by genetic alterations in neurotrophic receptor tyrosine kinase (NTRK) genes. These fusions result in constitutively active TRK fusion proteins that drive oncogenesis across multiple tumor types. The discovery of these fusions has catalyzed the development of targeted therapies, transforming the treatment landscape for eligible patients.
Understanding NTRK Gene Fusions in Solid Tumors
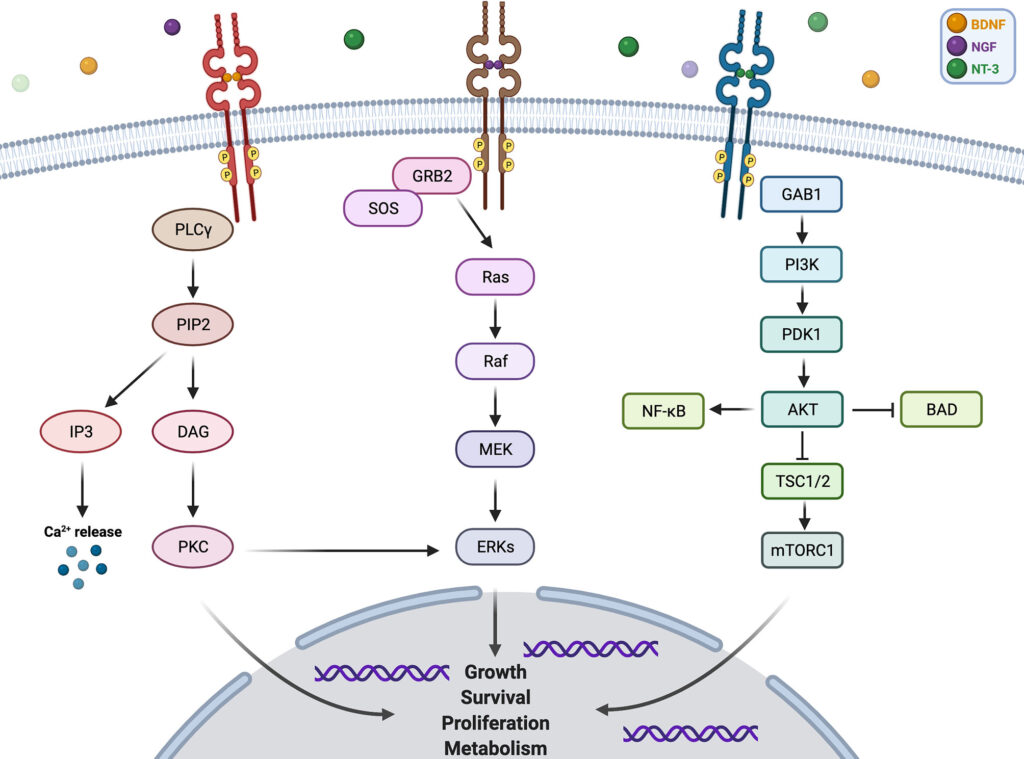
The NTRK1, NTRK2, and NTRK3 genes encode the TRKA, TRKB, and TRKC receptors, respectively. Under normal conditions, these receptors play roles in the development and function of the nervous system by mediating signals for cell survival, differentiation, and growth. When an NTRK gene fuses with another unrelated gene—often due to chromosomal rearrangements—a chimeric oncoprotein is formed. These fusion proteins exhibit ligand-independent kinase activation, triggering uncontrolled proliferation.
Common Fusion Partners:
- ETV6-NTRK3 (seen in infantile fibrosarcoma, secretory breast carcinoma)
- TPM3-NTRK1, LMNA-NTRK1, and others (in thyroid cancer, colon cancer, and lung cancer)
Tumor Types Associated with NTRK Gene Fusions
Though rare overall, NTRK fusions can occur in both common and rare tumor histologies. The frequency is higher in certain rare cancers, but they are also found at lower rates in more prevalent tumors.
| Tumor Type | Frequency of NTRK Fusion | Notes |
|---|---|---|
| Secretory breast carcinoma | ~90% | ETV6-NTRK3 hallmark |
| Infantile fibrosarcoma | ~90% | Diagnostic fusion driver |
| Thyroid carcinoma | 2–4% | Often in papillary subtype |
| Non-small cell lung cancer (NSCLC) | <1% | Rare but clinically actionable |
| Colorectal carcinoma | <1% | Found mostly in MSI-high tumors |
| Soft tissue sarcoma | Variable | Fusion-associated histologies |
Molecular Diagnosis of NTRK Gene Fusions
Diagnostic Approaches:
- IHC serves as a cost-effective screening tool.
- FISH (fluorescence in situ hybridization) and RT-PCR detect known fusion transcripts.
- NGS, especially RNA-based panels, is preferred for identifying novel or rare fusions.
Clinical Significance and Pathogenic Role of TRK Fusion Proteins
The TRK fusion proteins promote oncogenesis through:
- Continuous activation of downstream signaling pathways such as RAS/MAPK, PI3K/AKT, and PLCγ.
- Enhanced cellular proliferation, survival, angiogenesis, and resistance to apoptosis.
This oncogenic addiction creates a dependency on the TRK signaling axis, making these tumors highly responsive to TRK inhibitors.
TRK Inhibitors: Targeted Therapy Revolution
FDA-Approved TRK Inhibitors:
| Drug Name | Target(s) | Approved Indications | Notable Features |
|---|---|---|---|
| Larotrectinib | TRKA/B/C | NTRK fusion-positive solid tumors (regardless of site) | High response rates, well-tolerated |
| Entrectinib | TRKA/B/C, ROS1, ALK | NTRK fusion-positive solid tumors and ROS1+ NSCLC | CNS penetration, multi-target profile |
Both agents are approved under a tissue-agnostic indication, representing a major milestone in precision oncology.
Efficacy Outcomes:
- Larotrectinib: ORR ~75%, median duration of response >35 months
- Entrectinib: ORR ~57%, durable responses with intracranial activity
Resistance Mechanisms and Next-Generation Inhibitors
Despite initial success, acquired resistance may develop via:
- Kinase domain mutations (e.g., G595R, G667C in NTRK1)
- Bypass signaling activation (e.g., MET amplification)
Next-Generation Agents in Development:
- Selitrectinib and Repotrectinib are being investigated for overcoming resistance mutations with promising early-phase data.
Guidelines and Recommendations
- NCCN and ESMO recommend routine testing for NTRK fusions in certain cancers (e.g., thyroid, salivary gland, and soft tissue sarcomas).
- NTRK testing is advised for advanced, metastatic, or refractory tumors with no standard targeted options.
- Multidisciplinary tumor boards should evaluate candidates for NTRK fusion testing.
Future Perspectives in NTRK Fusion Oncology
- Pan-tumor trials are ongoing to expand indications for TRK inhibitors.
- Liquid biopsy innovations aim to detect NTRK fusions non-invasively through ctDNA.
- Research into combination therapies may address resistance and enhance durability of responses.
Frequently Asked Questions:
Is NTRK fusion common in all cancers?
No, it’s rare in most cancers but highly prevalent in select histologies like secretory carcinomas and infantile fibrosarcoma.
How is NTRK fusion testing performed?
Primarily through next-generation sequencing or immunohistochemistry, followed by confirmatory tests.
Are TRK inhibitors effective across all NTRK fusion tumors?
Yes, they are tissue-agnostic, provided the tumor is driven by a functional TRK fusion protein.
What are the side effects of TRK inhibitors?
Generally mild, including fatigue, dizziness, weight gain, and liver enzyme elevation. Most are manageable with supportive care.
Can resistance to TRK inhibitors be reversed?
Yes, next-generation inhibitors targeting resistance mutations are in development and show promising results.
Solid tumors with NTRK gene fusions represent a paradigm shift in oncology, allowing for effective, site-agnostic targeted therapies that significantly improve outcomes. Early detection through comprehensive genomic profiling and timely initiation of TRK inhibitor therapy are essential for optimal management. Continued research and clinical innovation promise to further refine treatment strategies and extend benefits to a broader patient population.