Severe Combined Immunodeficiency (SCID) represents a group of rare, life-threatening genetic disorders characterized by the profound impairment of both humoral and cellular immunity. Without early diagnosis and treatment, infants with SCID are vulnerable to recurrent, severe infections leading to high mortality within the first year of life.
SCID is widely recognized as a pediatric emergency. Timely identification and intervention—particularly hematopoietic stem cell transplantation (HSCT)—are critical for survival and long-term immune restoration.
Genetic Basis and Classification of SCID
SCID arises from various gene mutations that impair the development or function of T cells, and in many cases, B and NK cells. The disorder is primarily inherited in an X-linked or autosomal recessive pattern, depending on the specific mutation involved.
Common Genetic Subtypes of SCID:
| Subtype | Gene Affected | Inheritance Pattern | T Cells | B Cells | NK Cells |
|---|---|---|---|---|---|
| X-linked SCID | IL2RG | X-linked | Absent | Present | Absent |
| ADA deficiency | ADA | Autosomal recessive | Absent | Absent | Absent |
| JAK3 deficiency | JAK3 | Autosomal recessive | Absent | Present | Absent |
| RAG1/RAG2 deficiency | RAG1, RAG2 | Autosomal recessive | Absent | Absent | Present |
| IL-7Rα deficiency | IL7R | Autosomal recessive | Absent | Present | Present |
Pathophysiology: Mechanisms of Immune Dysfunction in SCID
SCID disrupts the normal maturation and function of lymphocytes:
- T-cell deficiency prevents the orchestration of the adaptive immune response.
- B-cell dysfunction limits antibody production, even when B cells are present.
- NK cell impairment reduces innate immunity and viral clearance.
Together, these deficits render the patient highly susceptible to a broad spectrum of pathogens—bacterial, viral, and fungal.
Clinical Presentation: Recognizing Symptoms of SCID
Infants with SCID typically appear normal at birth but begin exhibiting severe infections within the first few months of life. Key clinical features include:
- Recurrent pneumonia, otitis media, or candidiasis
- Persistent diarrhea
- Growth failure or wasting
- Lack of palpable lymph nodes or tonsils
- Adverse reactions to live vaccines (e.g., rotavirus, BCG)
Without functional lymphocytes, infections can become rapidly fatal, emphasizing the necessity of early detection.
Diagnostic Approach to SCID
Newborn Screening
In many countries, newborn screening for SCID is conducted via T-cell receptor excision circle (TREC) assay, a sensitive method to detect low or absent T cells.
Laboratory Tests
- Lymphocyte subset analysis: Flow cytometry to quantify T, B, and NK cells
- Immunoglobulin levels: Typically low or undetectable IgG, IgA, and IgM
- Genetic testing: Identification of the causative mutation for classification and family counseling
Treatment Strategies for SCID
Hematopoietic Stem Cell Transplantation (HSCT)
HSCT remains the definitive treatment for most SCID types and offers the best outcomes when performed before the onset of severe infections, ideally within the first 3.5 months of life.
Donor types include:
- HLA-matched sibling donor (MSD) – best survival outcomes
- Matched unrelated donor (MUD)
- Haploidentical parental donor with T-cell depletion
Enzyme Replacement Therapy (ERT)
For adenosine deaminase (ADA) deficiency, PEG-ADA enzyme therapy may serve as a temporary measure or bridge to transplant, restoring partial immune function.
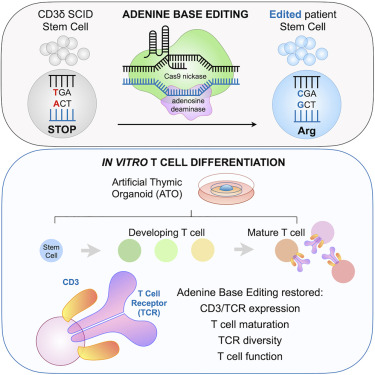
Gene Therapy
In selected SCID subtypes (notably IL2RG, ADA), gene therapy using autologous hematopoietic stem cells modified ex vivo with viral vectors has demonstrated promising outcomes.
Gene therapy advantages:
- Eliminates graft-versus-host disease risk
- Promotes endogenous immune reconstitution
- Particularly useful when a matched donor is unavailable
Prognosis and Long-Term Follow-Up
Prognosis strongly correlates with age at diagnosis, infection status at treatment, and donor match quality. With early HSCT, survival rates exceed 90% in many centers. Survivors require:
- Immunologic monitoring
- Vaccination updates (excluding live vaccines unless full immune reconstitution is confirmed)
- Lifelong surveillance for late effects such as autoimmune diseases, secondary cancers, or chronic lung disease
Preventive Measures and Family Counseling
Infection Prevention
- Protective isolation until immune function is restored
- Avoidance of live vaccines
- Prophylactic antimicrobials (e.g., TMP-SMX, antifungals)
Genetic Counseling and Prenatal Diagnosis
For families with a known SCID mutation:
- Carrier testing and genetic counseling are crucial
- Prenatal diagnosis via chorionic villus sampling or amniocentesis can inform pregnancy management
Research and Future Directions
Ongoing research is focused on improving gene editing techniques (e.g., CRISPR-Cas9), enhancing vector safety, and extending newborn screening globally. The future holds promise for:
- Universal SCID screening in all countries
- Safer, non-myeloablative conditioning regimens
- Expanded access to gene therapy in low-resource settings
Severe Combined Immunodeficiency (SCID) is a pediatric medical emergency requiring immediate diagnosis and treatment. Advances in HSCT, gene therapy, and early detection have significantly improved outcomes. Multidisciplinary care—encompassing immunologists, transplant specialists, geneticists, and primary care providers—is essential to ensure long-term survival and quality of life.