Rotavirus is a leading cause of severe gastroenteritis in infants and young children worldwide. Characterized by profuse diarrhea, vomiting, fever, and dehydration, rotavirus infection results in significant morbidity and mortality, particularly in low-income countries. Prior to the introduction of the rotavirus vaccine, the virus accounted for more than 450,000 child deaths annually.
Rotavirus vaccination represents one of the most effective public health interventions to prevent severe diarrheal disease and its complications in children under five.
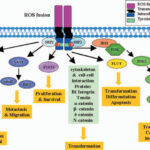
Understanding the Rotavirus Pathogen
Rotavirus is a double-stranded RNA virus belonging to the Reoviridae family. It primarily spreads via the fecal-oral route and exhibits high environmental resilience, allowing it to persist on surfaces and in water supplies.
Key features of the virus include:
- High infectivity with a low infectious dose
- Short incubation period of 1–3 days
- Multiple serotypes, with G1P[8] being the most prevalent globally
- Frequent reinfections, although subsequent episodes are typically less severe
Global Burden of Rotavirus Infection
Before widespread vaccination:
- Nearly every child globally was infected by age 5
- Hospitalizations for rotavirus-related diarrhea were common in high- and middle-income countries
- Deaths were concentrated in low-income nations with limited access to healthcare
With vaccine introduction, there has been a notable decline in rotavirus-related morbidity and mortality, validating the importance of immunization strategies.
Available Rotavirus Vaccines
Two main oral, live-attenuated vaccines are used globally:
1. Rotarix (RV1) – GlaxoSmithKline
- Monovalent vaccine (G1P[8])
- Administered in a two-dose series
- Typically given at 6 and 10 weeks of age
2. RotaTeq (RV5) – Merck
- Pentavalent vaccine (G1, G2, G3, G4, and P[8])
- Administered in a three-dose series
- Typically given at 6, 10, and 14 weeks of age
Other vaccines in use or under evaluation include:
- Rotavac – Bharat Biotech (India)
- Rotasiil – Serum Institute of India
- Lanzhou Lamb Rotavirus Vaccine – China
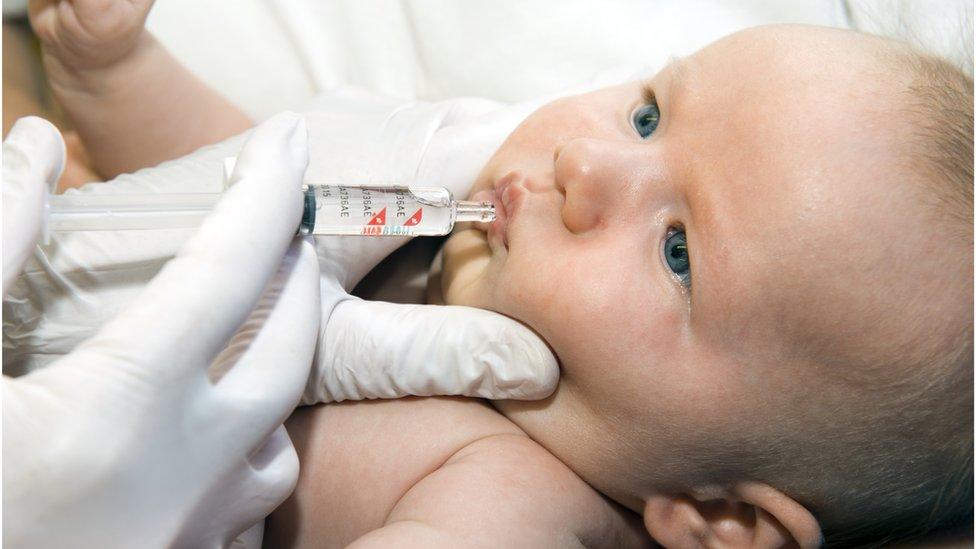
Rotavirus Vaccine Schedule and Administration Guidelines
The World Health Organization (WHO) recommends rotavirus vaccination as part of the routine immunization schedule. The schedule may vary slightly by country but generally adheres to the following timeline:
Key considerations:
- Vaccination must begin before 15 weeks of age
- Final dose should be administered by 8 months
- Oral administration enhances compliance and avoids needle use
Effectiveness of Rotavirus Vaccination
Numerous studies have validated the high efficacy of rotavirus vaccines:
- High-income countries report 85–98% protection against severe rotavirus gastroenteritis
- Middle- and low-income regions see efficacy around 50–64%, still yielding substantial public health benefits
- Marked reduction in hospitalizations and emergency visits observed post-vaccine introduction
Indirect benefits, such as herd immunity, have also been reported in vaccinated populations.
Safety and Side Effects
Rotavirus vaccines have an excellent safety profile. Common and generally mild side effects include:
- Irritability
- Mild, temporary diarrhea or vomiting
- Fever
Intussusception Risk
A small increased risk of intussusception (a type of bowel obstruction) has been associated with rotavirus vaccination, typically within 7 days of the first dose. However, the benefits of vaccination far outweigh this risk. Global health authorities continue to support routine rotavirus immunization.
Contraindications and Precautions
Rotavirus vaccines should not be administered to:
- Infants with a history of severe allergic reaction to previous doses
- Infants with severe combined immunodeficiency (SCID)
- Infants with a history of intussusception
Vaccination can generally proceed in mildly ill children but should be deferred during moderate to severe illness.
Integration Into Global Immunization Programs
As of 2024, more than 110 countries have integrated rotavirus vaccines into national immunization schedules. This integration has been supported by:
- Global Alliance for Vaccines and Immunization (Gavi)
- WHO prequalification and recommendations
- Surveillance systems monitoring vaccine impact and safety
Regions such as Africa, Latin America, and Southeast Asia have observed dramatic reductions in diarrheal mortality post-introduction.
Public Health Impact and Outcomes
The introduction of rotavirus vaccines has yielded multiple public health achievements:
- 50–70% reduction in rotavirus hospitalizations globally
- Significant declines in diarrhea-related deaths among children under five
- Reduced economic burden on healthcare systems
- Improved quality of life for children and caregivers
Continued vaccine coverage and monitoring are vital to sustaining these gains.
Addressing Challenges in Vaccine Uptake
Despite progress, some regions face barriers to rotavirus vaccination:
- Cold chain logistics for oral vaccines
- Cultural resistance or vaccine hesitancy
- Inconsistent supply chains in resource-limited settings
To improve uptake, efforts must include:
- Community education and outreach
- Enhanced surveillance and evaluation
- Sustainable financing mechanisms
Rotavirus vaccination has transformed the global fight against diarrheal diseases in children. With robust safety, proven efficacy, and substantial health and economic benefits, rotavirus vaccines should remain a cornerstone of pediatric public health programs. Achieving universal vaccine coverage, particularly in underserved regions, is essential to eliminating rotavirus-associated mortality and reducing disease burden worldwide.